INTRODUCTION The HSAB concept is an acronym for `hard and soft
... likely to form oxides, carbonates, nitrides and fluorides, while Type B metals are more likely to form phosphides, sulfides and selinides. This type of analysis is of great economic importance because some metals are found in nature as sulfide ores: PbS, CdS, NiS, etc., while other are found as carb ...
... likely to form oxides, carbonates, nitrides and fluorides, while Type B metals are more likely to form phosphides, sulfides and selinides. This type of analysis is of great economic importance because some metals are found in nature as sulfide ores: PbS, CdS, NiS, etc., while other are found as carb ...
RULES OF CHEMICAL NOMENCLATURE I. Elements (periodic
... are disolved in water (aqueous or “aq”) 1. Can be named as salts if not dissolved in water 2. Binary Acids: hydrogen + non-metal a. named by changing hydrogen to “hydro” b. change non-metal ending to “ic” (change “ide” to “ic”) c. followed by the word “acid” (ex: HCl = hydrochloric acid) 3. Ternary ...
... are disolved in water (aqueous or “aq”) 1. Can be named as salts if not dissolved in water 2. Binary Acids: hydrogen + non-metal a. named by changing hydrogen to “hydro” b. change non-metal ending to “ic” (change “ide” to “ic”) c. followed by the word “acid” (ex: HCl = hydrochloric acid) 3. Ternary ...
Chapter 2 - Molecules of Life (Biochemistry) Periodic Table of
... • Electrons not shared equally! • One atom “hogs” the electrons! • This leads to the formation of hydrogen bonds.! ...
... • Electrons not shared equally! • One atom “hogs” the electrons! • This leads to the formation of hydrogen bonds.! ...
Chapter 3
... Once again, charges MUST be observed when recombining and formulas are written with cation first. Reaction only happens if one of the two products is Insoluble (s). LEP #14 a, b ...
... Once again, charges MUST be observed when recombining and formulas are written with cation first. Reaction only happens if one of the two products is Insoluble (s). LEP #14 a, b ...
Chemistry to Remember
... creation of ions that are electrically charged particles with properties totally different from the atom from which they came. Covalence is sharing pairs of electrons via a single, double, or triple bond. Radicals are clusters of elements held together by covalent bonds that behave as if they were a ...
... creation of ions that are electrically charged particles with properties totally different from the atom from which they came. Covalence is sharing pairs of electrons via a single, double, or triple bond. Radicals are clusters of elements held together by covalent bonds that behave as if they were a ...
Chemistry - SchoolNotes.com
... 54) How does shielding affect the ionization energy? 55) How many valence electrons are there in an atom of phosphorus? 5 56) What is the electron configuration of the calcium ion, Ca2+? 1s22s22p63s23p6 57) How many electrons does barium have to give up to achieve a noble-gas electron configuration? ...
... 54) How does shielding affect the ionization energy? 55) How many valence electrons are there in an atom of phosphorus? 5 56) What is the electron configuration of the calcium ion, Ca2+? 1s22s22p63s23p6 57) How many electrons does barium have to give up to achieve a noble-gas electron configuration? ...
Exam Review
... 21. Compared to the stability of the original atom, the stability of its ion that resembles a noble gas configuration would be a) identical b) sometimes less c) less d) greater 22. The formation of bonds between atoms depends on __. a) the electron configurations of the atoms involved c) both of the ...
... 21. Compared to the stability of the original atom, the stability of its ion that resembles a noble gas configuration would be a) identical b) sometimes less c) less d) greater 22. The formation of bonds between atoms depends on __. a) the electron configurations of the atoms involved c) both of the ...
Non-Metals
... Nitrogen is notoriously inert . However it will combine with oxygen at high temperatures to form nitrous oxides . This occurs during lightning discharges and in the engines of vehicles . It will also combine with hydrogen under certain conditions of temperature and pressure to form ammonia . ...
... Nitrogen is notoriously inert . However it will combine with oxygen at high temperatures to form nitrous oxides . This occurs during lightning discharges and in the engines of vehicles . It will also combine with hydrogen under certain conditions of temperature and pressure to form ammonia . ...
- Cypress HS
... then be allowed to stand for one week to come to equilibrium. As the reactants react the acidity of the mixture will decrease, reaching a minimum once the system reaches equilibrium. You will be able to qualitatively be able to measure this with your sense of smell. Week one the acetic acid is stron ...
... then be allowed to stand for one week to come to equilibrium. As the reactants react the acidity of the mixture will decrease, reaching a minimum once the system reaches equilibrium. You will be able to qualitatively be able to measure this with your sense of smell. Week one the acetic acid is stron ...
Reactions of Metals and Their Compounds
... piece of zinc(Zn) in a small beaker or petri dish Collect 5mL of limewater(Ca(OH)2) in a test tube. Gently blow through a straw into the test tube until a change occurs. ...
... piece of zinc(Zn) in a small beaker or petri dish Collect 5mL of limewater(Ca(OH)2) in a test tube. Gently blow through a straw into the test tube until a change occurs. ...
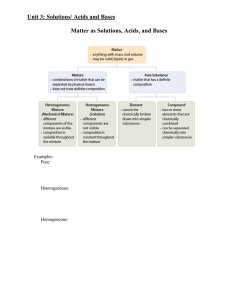
Outline for Unit 1 Solutions, Acid/Base, and Gases
... Ionization/Dissociation: when acidic molecular substances or soluble ionic substances dissolve(dissociate) in water, they ionize or form ions. Page 213 questions ...
... Ionization/Dissociation: when acidic molecular substances or soluble ionic substances dissolve(dissociate) in water, they ionize or form ions. Page 213 questions ...
Solution - gearju.com
... What are the characteristics of a Brønsted acid? Does it contain at least an H atom? With the exception of ammonia, most Brønsted bases that you will encounter at this stage are anions. ...
... What are the characteristics of a Brønsted acid? Does it contain at least an H atom? With the exception of ammonia, most Brønsted bases that you will encounter at this stage are anions. ...
A2 2, Analytical, Transition Metals, Electrochemistry and
... breathing carbon monoxide can result in death. ...
... breathing carbon monoxide can result in death. ...
09 Stoichiometry WS Stoichiometry WS
... 1. How many moles of ammonium sulfate can be made from the reaction of 30.0 mol of NH3 with H2SO4 according to the following equation? 2NH3 + H2SO4 (NH4)2SO4 2. In a very violent reaction called a thermite reaction, aluminum metal reacts with iron(III) oxide to form iron metal and aluminum oxide acc ...
... 1. How many moles of ammonium sulfate can be made from the reaction of 30.0 mol of NH3 with H2SO4 according to the following equation? 2NH3 + H2SO4 (NH4)2SO4 2. In a very violent reaction called a thermite reaction, aluminum metal reacts with iron(III) oxide to form iron metal and aluminum oxide acc ...
Name……………………………………............................. Index number
... (b)Use dots ( ) and crosses (x) to show bonding in the compound formed in (a) above. (1mark) ...
... (b)Use dots ( ) and crosses (x) to show bonding in the compound formed in (a) above. (1mark) ...
AP Chem Equations - Speedway High School
... Acids react with bases to produce salts and water. One mole of hydrogen ions react with one mole of hydroxide ions to produce one mole of water. ...
... Acids react with bases to produce salts and water. One mole of hydrogen ions react with one mole of hydroxide ions to produce one mole of water. ...
WRITING AP EQUATIONS AP equation sets are found in the free
... Acids react with bases to produce salts and water. One mole of hydrogen ions react with one mole of hydroxide ions to produce one mole of water. ...
... Acids react with bases to produce salts and water. One mole of hydrogen ions react with one mole of hydroxide ions to produce one mole of water. ...
Chemistry - StudyTime NZ
... Acids are characterised by having excess Hydroxide (OH-) groups attached to them. These hydroxide groups can be used to accept protons and form water (H2O) ...
... Acids are characterised by having excess Hydroxide (OH-) groups attached to them. These hydroxide groups can be used to accept protons and form water (H2O) ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.