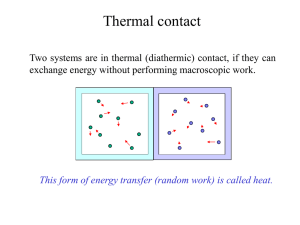
λ - Chemistry 7
... MYSTERY #3: Narrow bands of colors of light are emitted when gases are excited by high voltage. The colors are characteristic and reproducible for different elements. The light generated by the ...
... MYSTERY #3: Narrow bands of colors of light are emitted when gases are excited by high voltage. The colors are characteristic and reproducible for different elements. The light generated by the ...
chapter 5 energy, matter, and momentum exchanges near the surface
... The troposphere is characterized by temperatures that normally decrease with height both because of the decreasing compression of atmospheric gases with increasing distance from the surface and because of the increasing distance from the (indirect) heat source – the surface The Near-Surface Trop ...
... The troposphere is characterized by temperatures that normally decrease with height both because of the decreasing compression of atmospheric gases with increasing distance from the surface and because of the increasing distance from the (indirect) heat source – the surface The Near-Surface Trop ...
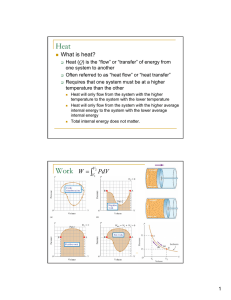
Heat Work
... A P-V diagram for a reversible heat engine in which 1.00 mole of argon, a nearly ideal monatomic gas, is initially at STP (point a). Points b and c are on an isothermal. If the engine produces positive work, a) is the cycle clockwise or counter clockwise, b) what is the efficiency of the cycle? ...
... A P-V diagram for a reversible heat engine in which 1.00 mole of argon, a nearly ideal monatomic gas, is initially at STP (point a). Points b and c are on an isothermal. If the engine produces positive work, a) is the cycle clockwise or counter clockwise, b) what is the efficiency of the cycle? ...
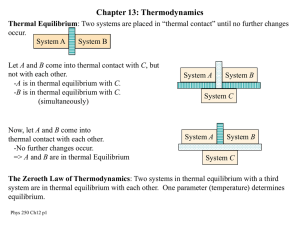
p250c13
... Example: A heat pump is used to maintain an inside temperature of 20 ºC when the outside temperature is 10ºC. What is the theoretical maximum cp for this heat pump? If the pump is to deliver heat at a rate of 15 kW, how much power must be supplied to run the pump? ...
... Example: A heat pump is used to maintain an inside temperature of 20 ºC when the outside temperature is 10ºC. What is the theoretical maximum cp for this heat pump? If the pump is to deliver heat at a rate of 15 kW, how much power must be supplied to run the pump? ...
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
Vocabulary - cloudfront.net
... 4. A 50 gram piece of aluminum is heated to 100C and then dropped into cool water where the aluminum’s temperature drops to 30C. How many calories does the aluminum loose to the water? (Specific heat capacity Al = 0.215 cal/gC). ...
... 4. A 50 gram piece of aluminum is heated to 100C and then dropped into cool water where the aluminum’s temperature drops to 30C. How many calories does the aluminum loose to the water? (Specific heat capacity Al = 0.215 cal/gC). ...
Physics, Chapter 18: Transfer of Heat
... ft hroF' The thermal conductivities of metals are generally greater than those of other solids, and silver is the best conductor of all. It is also interesting to note that those substances which are good conductors of heat are also good conductors of electricity. The thermal conductivities of some ...
... ft hroF' The thermal conductivities of metals are generally greater than those of other solids, and silver is the best conductor of all. It is also interesting to note that those substances which are good conductors of heat are also good conductors of electricity. The thermal conductivities of some ...
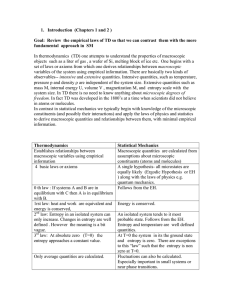
Topic 2 The first law of thermodynamics
... Extensive properties: dependent on amount Intensive properties: independent on amount ...
... Extensive properties: dependent on amount Intensive properties: independent on amount ...
Physics 334 Modern Physics
... This is known as the Stefan-Boltzmann law, with the constant σ experimentally measured to be 5.6705 × 10−8 W / (m2 · K4). The emissivity є (є = 1 for an idealized blackbody) is simply the ratio of the emissive power of an object to that of an ideal blackbody and is always less than 1. ...
... This is known as the Stefan-Boltzmann law, with the constant σ experimentally measured to be 5.6705 × 10−8 W / (m2 · K4). The emissivity є (є = 1 for an idealized blackbody) is simply the ratio of the emissive power of an object to that of an ideal blackbody and is always less than 1. ...
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.