Electronic Structure - Chemistry Teaching Resources
... excited shows four lines: red, blue-green, blue and indigo ...
... excited shows four lines: red, blue-green, blue and indigo ...
Lecture 6/7 - TCD Chemistry
... Heat cannot be detected or measured directly. There is no ‘heat meter’. One way to determine the magnitude of a heat transfer is to measure the work needed to bring about the same change in the thermodynamic state of a system as was produced by heat transfer. Another approach is to deduce the magnit ...
... Heat cannot be detected or measured directly. There is no ‘heat meter’. One way to determine the magnitude of a heat transfer is to measure the work needed to bring about the same change in the thermodynamic state of a system as was produced by heat transfer. Another approach is to deduce the magnit ...
Heat Capacity - Uplift North Hills Prep
... • Peak wavelength of light emitted depends on temperature • Spectrum includes all wavelength longer than the peak but not many above – 20°C - peak in infrared (need thermal imaging camera to see body heat) – 800°C - peak in red (electric coil, fire glows reds) ...
... • Peak wavelength of light emitted depends on temperature • Spectrum includes all wavelength longer than the peak but not many above – 20°C - peak in infrared (need thermal imaging camera to see body heat) – 800°C - peak in red (electric coil, fire glows reds) ...
chapter20
... An isovolumetric process is one in which there is no change in the volume Since the volume does not change, W = 0 From the first law, DEint = Q If energy is added by heat to a system kept at constant volume, all of the transferred energy remains in the system as an increase in its internal energy ...
... An isovolumetric process is one in which there is no change in the volume Since the volume does not change, W = 0 From the first law, DEint = Q If energy is added by heat to a system kept at constant volume, all of the transferred energy remains in the system as an increase in its internal energy ...
How to quickly cool a bottle of drink
... coefficients of both the drink and the air, as both offer resistance for the energy to get transferred as heat from the drink to the air. For the sake of brevity of this post, let us take it for granted that hdrink hair ...
... coefficients of both the drink and the air, as both offer resistance for the energy to get transferred as heat from the drink to the air. For the sake of brevity of this post, let us take it for granted that hdrink hair ...
ENGINEERING_THERMODYNAMICS
... system at a higher-level temperature to a system at a lower level temperature. It can never happen in the reverse direction by nature. This contrary will be discussed in the Second law of thermodynamics. Heat is measured in Joules or Kilo Joules. Heat can only be sensed due to the temperature differ ...
... system at a higher-level temperature to a system at a lower level temperature. It can never happen in the reverse direction by nature. This contrary will be discussed in the Second law of thermodynamics. Heat is measured in Joules or Kilo Joules. Heat can only be sensed due to the temperature differ ...
The design of high-temperature thermal conductivity measurements
... temperature because the analysis will be complicated if the differences of temperature are involved in the analysis [2]. However, for certain materials particularly for thermoelectric material, the differences of thermal conductivity should not be ignored because thermoelectric operates at high temp ...
... temperature because the analysis will be complicated if the differences of temperature are involved in the analysis [2]. However, for certain materials particularly for thermoelectric material, the differences of thermal conductivity should not be ignored because thermoelectric operates at high temp ...
Lecture 4: 09.16.05 Temperature, heat, and entropy
... o� Why would two metals heated to the same temperature have a different ability to melt the wax strip? The answer lies in the equation above relating temperature and heat, which indicates that two metals at the same temperature have not necessarily received the same amount of energysince the amount ...
... o� Why would two metals heated to the same temperature have a different ability to melt the wax strip? The answer lies in the equation above relating temperature and heat, which indicates that two metals at the same temperature have not necessarily received the same amount of energysince the amount ...
Chapter 2. Entropy and Temperature
... The fundamental assumption of statistical mechanics is that a closed system in equilibrium is equally likely to be in any one of the (quantum) states accessible to it. A closed system has no contact with any other system and so has fixed total energy, number of particles, volume and constant values f ...
... The fundamental assumption of statistical mechanics is that a closed system in equilibrium is equally likely to be in any one of the (quantum) states accessible to it. A closed system has no contact with any other system and so has fixed total energy, number of particles, volume and constant values f ...
Reading - 1st Law of Thermodynamics
... Pressure/Volume Work: Many engines that do work involve pistons and cylinders. Everybody know what they are? A cylinder is tube and a piston is a solid cylindrical device that exactly fits into the cylinder so that there is very little gap between the piston and the side of the cylinder. ...
... Pressure/Volume Work: Many engines that do work involve pistons and cylinders. Everybody know what they are? A cylinder is tube and a piston is a solid cylindrical device that exactly fits into the cylinder so that there is very little gap between the piston and the side of the cylinder. ...
ME6301- ENGINEERING THERMODYNAMICS UNIT – I BASIC
... 1. State the Kelvin- Planck statement of the second law of thermodynamics. (N/D 2006) Kelvin-Planck states that it is impossible to construct a heat engine working on cyclic process, whose only purpose is to convert all the heat energy given to it in an equal amount of work. 2. Write a short note on ...
... 1. State the Kelvin- Planck statement of the second law of thermodynamics. (N/D 2006) Kelvin-Planck states that it is impossible to construct a heat engine working on cyclic process, whose only purpose is to convert all the heat energy given to it in an equal amount of work. 2. Write a short note on ...
Chapter 1: FUNDAMENTAL CONCEPTS OF THERMODYNAMICS
... Thermodynamics is that branch of science which deals with energy transfer A system may be closed, open or isolated system A homogeneous system is one which consists of a single phase A heterogeneous system is one which consists of two or more phases. A property of a system is a characteristic of the ...
... Thermodynamics is that branch of science which deals with energy transfer A system may be closed, open or isolated system A homogeneous system is one which consists of a single phase A heterogeneous system is one which consists of two or more phases. A property of a system is a characteristic of the ...
Thermodynamics for Systems Biology
... The first law expresses the conservation of energy. The law was constructed from separate older conservation laws for mechanical energy in mechanics and for caloric in the theory of heat. It retrospect, the ability to rub two sticks together to produce heat flagrantly contradicts any conservation of ...
... The first law expresses the conservation of energy. The law was constructed from separate older conservation laws for mechanical energy in mechanics and for caloric in the theory of heat. It retrospect, the ability to rub two sticks together to produce heat flagrantly contradicts any conservation of ...
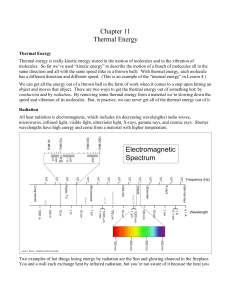
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.