Thermodynamics: Notes
... The rest of the universe outside our system we call the surroundings. The system and the surroundings are separated by a boundary or a wall. They may, in general, exchange energy and matter, depending on the nature of the wall. A closed system is one where there is no exchange of matter. An equilibr ...
... The rest of the universe outside our system we call the surroundings. The system and the surroundings are separated by a boundary or a wall. They may, in general, exchange energy and matter, depending on the nature of the wall. A closed system is one where there is no exchange of matter. An equilibr ...
heat processes
... EGM is a design concept based upon minimization of irreversible processes. It is a new philosophy: reversible processes are good, irreversible wrong. As a measure of irreversibility the rate of entropy generation in a system is considered. Entropy increase is caused by heat transfer from high to low ...
... EGM is a design concept based upon minimization of irreversible processes. It is a new philosophy: reversible processes are good, irreversible wrong. As a measure of irreversibility the rate of entropy generation in a system is considered. Entropy increase is caused by heat transfer from high to low ...
Rad Measurements Instrument Instructor Notes
... they were originated and reduce the electric field to a point where further avalanche is impossible. ...
... they were originated and reduce the electric field to a point where further avalanche is impossible. ...
VNIR Reflectance Spectroscopy
... NIR: 0.75 - 3 µm Mid-infrared: 3 - 8 µm Thermal infrared : 4 - 50 µm ...
... NIR: 0.75 - 3 µm Mid-infrared: 3 - 8 µm Thermal infrared : 4 - 50 µm ...
Q - Department of Applied Physics
... where Q is heat. This is the mathematical statement of the first law. This means that the internal energy can be increased either by doing work on or by supplying heat to the system. It is true for all processes whether reversible or irreversible. In closed systems, heat is the non-mechanical exchan ...
... where Q is heat. This is the mathematical statement of the first law. This means that the internal energy can be increased either by doing work on or by supplying heat to the system. It is true for all processes whether reversible or irreversible. In closed systems, heat is the non-mechanical exchan ...
Chapter 4 Entropy and second law of thermodynamics
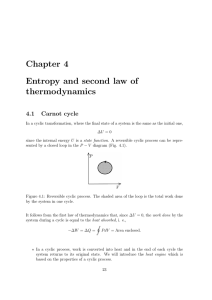
... From the microscopic point of view - heat transfer is an exchange of energy due to the random motion of atoms; - work ’s performance requires an organized action of atoms. In these terms, heat being converted entirely into work means chaos changing spontaneously to order, which is a very improbable ...
... From the microscopic point of view - heat transfer is an exchange of energy due to the random motion of atoms; - work ’s performance requires an organized action of atoms. In these terms, heat being converted entirely into work means chaos changing spontaneously to order, which is a very improbable ...
Document
... • If HEAT IS ADDED to the system (gas) è Q > 0 • If HEAT IS RELEASED by the system (gas) è Q < 0 W is the work • If WORK IS DONE ON the system (gas) è gas compresses è W > 0 • If WORK IS DONE BY the system (gas) è gas expands è W < 0 ...
... • If HEAT IS ADDED to the system (gas) è Q > 0 • If HEAT IS RELEASED by the system (gas) è Q < 0 W is the work • If WORK IS DONE ON the system (gas) è gas compresses è W > 0 • If WORK IS DONE BY the system (gas) è gas expands è W < 0 ...
Thermal Energy
... are said to be in thermal equilibrium. As long as they are isolated from other objects and cannot exchange any heat with their surroundings, they will remain at that temperature. For example, a thermos bottle filled with warm juice and ice cubes that melt, will arrive at some intermediate temperatur ...
... are said to be in thermal equilibrium. As long as they are isolated from other objects and cannot exchange any heat with their surroundings, they will remain at that temperature. For example, a thermos bottle filled with warm juice and ice cubes that melt, will arrive at some intermediate temperatur ...
IR Spectroscopy
... This absorption overlaps the sharper C-H stretching peaks, which may be seen extending beyond the O-H envelope at 2990, 2950 and 2870 cm-1. The smaller peaks protruding near 2655 and 2560 are characteristic of the ...
... This absorption overlaps the sharper C-H stretching peaks, which may be seen extending beyond the O-H envelope at 2990, 2950 and 2870 cm-1. The smaller peaks protruding near 2655 and 2560 are characteristic of the ...
Calorimetry Measurement
... there is a surplus of thermal energy (i.e., of fast molecules or electrons, or a higher density of phonons) in some area, some of it will flow toward areas with a lower thermal energy density until thermal equilibrium has been established. This flow of energy P (in W = J s–1) is called heat flow. He ...
... there is a surplus of thermal energy (i.e., of fast molecules or electrons, or a higher density of phonons) in some area, some of it will flow toward areas with a lower thermal energy density until thermal equilibrium has been established. This flow of energy P (in W = J s–1) is called heat flow. He ...
Lecture 10
... August 24, 1888), was a German physicist and mathematician, and was one of thefounders of thermodynamics. His most important paper, on the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. He ...
... August 24, 1888), was a German physicist and mathematician, and was one of thefounders of thermodynamics. His most important paper, on the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. He ...
Unit 12 - HKU Physics
... 12.12 The third law of thermodynamics The third law of thermodynamics states that there is no temperature lower than absolute zero, and that absolute zero is unattainable. It is possible to cool an object to temperatures arbitrarily close to absolute zero – experiments have reached temperatures as l ...
... 12.12 The third law of thermodynamics The third law of thermodynamics states that there is no temperature lower than absolute zero, and that absolute zero is unattainable. It is possible to cool an object to temperatures arbitrarily close to absolute zero – experiments have reached temperatures as l ...
chapter20
... Internal Energy and Other Energies The kinetic energy due to its motion through space is not included. ...
... Internal Energy and Other Energies The kinetic energy due to its motion through space is not included. ...
Thermodynamics - Atmosphere Physics
... No process is possible which results in the extraction of an amount of heat from a reservoir and its conversion to an equal amount of mechanical work. ...
... No process is possible which results in the extraction of an amount of heat from a reservoir and its conversion to an equal amount of mechanical work. ...
Lifting Fingerprints with Powders and Chemicals
... print work. Therefore, the black powder, composed of black carbon or charcoal, is applied to white or light-colored surfaces. The gray powder, composed basically of an aluminum dust, is used on dark-colored surfaces; it is also applied to mirrors and metal surfaces that are polished to a mirror-like ...
... print work. Therefore, the black powder, composed of black carbon or charcoal, is applied to white or light-colored surfaces. The gray powder, composed basically of an aluminum dust, is used on dark-colored surfaces; it is also applied to mirrors and metal surfaces that are polished to a mirror-like ...
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.