The first and second law of Thermodynamics - Ole Witt
... This work is, however, reversible, since it is the precisely equal to the work which has to be done to bring the gas back to its initial state, releasing the heat Q to the external reservoir. It is rather easy to convince yourself that Wirr < Wrev. Thus if we let the external pressure Pext be less t ...
... This work is, however, reversible, since it is the precisely equal to the work which has to be done to bring the gas back to its initial state, releasing the heat Q to the external reservoir. It is rather easy to convince yourself that Wirr < Wrev. Thus if we let the external pressure Pext be less t ...
PPT
... Even though we imagined a reversible process to substitute for dq, this equation applies to both reversible and irreversible transitions because they are state variables ...
... Even though we imagined a reversible process to substitute for dq, this equation applies to both reversible and irreversible transitions because they are state variables ...
File
... Conduction, Energy Through Solids In solids, where the particles are closely packed together, thermal energy can be transferred from one particle to another very easily. Thermal conduction is the process of transferring thermal energy by the direct collisions of the particles. The space between the ...
... Conduction, Energy Through Solids In solids, where the particles are closely packed together, thermal energy can be transferred from one particle to another very easily. Thermal conduction is the process of transferring thermal energy by the direct collisions of the particles. The space between the ...
18. Chemical Thermodynamics
... states that one form of energy disappears, an equivalent amount of another form of energy is produced. But it is salient about the extent to which such conversion can take place. 2. It does not tell about the direction of flow of heat. 3. It does not tell about spontaneity of reaction. ...
... states that one form of energy disappears, an equivalent amount of another form of energy is produced. But it is salient about the extent to which such conversion can take place. 2. It does not tell about the direction of flow of heat. 3. It does not tell about spontaneity of reaction. ...
Module - 1: Thermodynamics
... relevant part of the kitchen as the environment (with temperature TE) of that system. Our observation is that if TS is not equal to TE, then TS will change until the two temperatures are equal and thus thermal equilibrium is reached. Such a change in temperature is due to the transfer of energy betw ...
... relevant part of the kitchen as the environment (with temperature TE) of that system. Our observation is that if TS is not equal to TE, then TS will change until the two temperatures are equal and thus thermal equilibrium is reached. Such a change in temperature is due to the transfer of energy betw ...
thermodynamics
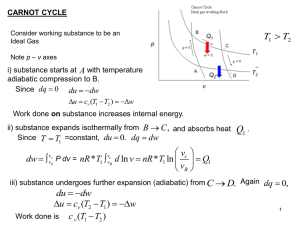
... 28. What is adiabatic process? 29. When does gas absorb heat and does work during isothermal process? 30. When does gas release heat and work is done by the surrounding? 31. Mention the condition for work done by the gas in an adiabatic process. 32. Mention the condition for work done on the gas in ...
... 28. What is adiabatic process? 29. When does gas absorb heat and does work during isothermal process? 30. When does gas release heat and work is done by the surrounding? 31. Mention the condition for work done by the gas in an adiabatic process. 32. Mention the condition for work done on the gas in ...
HEAT - Weebly
... relevant part of the kitchen as the environment (with temperature TE) of that system. Our observation is that if TS is not equal to TE, then TS will change until the two temperatures are equal and thus thermal equilibrium is reached. Such a change in temperature is due to the transfer of energy betw ...
... relevant part of the kitchen as the environment (with temperature TE) of that system. Our observation is that if TS is not equal to TE, then TS will change until the two temperatures are equal and thus thermal equilibrium is reached. Such a change in temperature is due to the transfer of energy betw ...
Word document format
... currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the system from the surroundings, q is positive. If one were carrying out the reaction i ...
... currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the system from the surroundings, q is positive. If one were carrying out the reaction i ...
Thermal Flux through a Surface of n-Octane. A Non
... We show using non-equilibrium molecular dynamics that there is local equilibrium in the surface when a two-phase fluid of n-octane is exposed to a large temperature gradient (108 K/m). The surface is defined according to Gibbs, and the transport across the surface is described with non-equilibrium t ...
... We show using non-equilibrium molecular dynamics that there is local equilibrium in the surface when a two-phase fluid of n-octane is exposed to a large temperature gradient (108 K/m). The surface is defined according to Gibbs, and the transport across the surface is described with non-equilibrium t ...
First Law of Thermodynamics - Derry Area School District
... When you look at large systems like the ideal gas with 1023 particles, the most likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different t ...
... When you look at large systems like the ideal gas with 1023 particles, the most likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different t ...
plumbum thiogallate optical properties
... than 0.7 g. The following process was used to homogenize the composition of the charge in the samples. From plumbum sulfide charge and gallium sulfide charge we prepared a charge of specified composition x at this temperature and subsequent cooling in the switched off furnace. The synthesized charge ...
... than 0.7 g. The following process was used to homogenize the composition of the charge in the samples. From plumbum sulfide charge and gallium sulfide charge we prepared a charge of specified composition x at this temperature and subsequent cooling in the switched off furnace. The synthesized charge ...
Sorption properties of sodium bicarbonate
... knowledge of parameters describing the structure of sorbents, such as surface area or pore size distribution play an important role for application them in chemical, cement industries, or modification of mineral raw materials. The sorption properties are determined by surface area, what binds with s ...
... knowledge of parameters describing the structure of sorbents, such as surface area or pore size distribution play an important role for application them in chemical, cement industries, or modification of mineral raw materials. The sorption properties are determined by surface area, what binds with s ...
Chapter 5 - Clayton State University
... energy that accompany chemical and physical processes. address 3 fundamental questions ...
... energy that accompany chemical and physical processes. address 3 fundamental questions ...
MET -303 THERMAL ENGINNERING-1 CHAPTER 1:
... It is a process of heat transfer from one particle of a body to another in the direction of fall of temperature. For example heat transfer through solids is by conduction. 2. Convection : The process of heat transfer from one particle to another by convection currents i.e. transfer of heat between t ...
... It is a process of heat transfer from one particle of a body to another in the direction of fall of temperature. For example heat transfer through solids is by conduction. 2. Convection : The process of heat transfer from one particle to another by convection currents i.e. transfer of heat between t ...
Chapter 1
... Internal Energy: The Internal Energy (U) of a system is the total energy content of the system. o It is the sum of the kinetic, potential, chemical, electrical, and all other forms of energy possessed by the atoms and molecules of the system. o The Internal Energy (U) is path independent and depends ...
... Internal Energy: The Internal Energy (U) of a system is the total energy content of the system. o It is the sum of the kinetic, potential, chemical, electrical, and all other forms of energy possessed by the atoms and molecules of the system. o The Internal Energy (U) is path independent and depends ...
Chapter 1 - All Made Easy
... Internal Energy: The Internal Energy (U) of a system is the total energy content of the system. o It is the sum of the kinetic, potential, chemical, electrical, and all other forms of energy possessed by the atoms and molecules of the system. o The Internal Energy (U) is path independent and depends ...
... Internal Energy: The Internal Energy (U) of a system is the total energy content of the system. o It is the sum of the kinetic, potential, chemical, electrical, and all other forms of energy possessed by the atoms and molecules of the system. o The Internal Energy (U) is path independent and depends ...
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. An object with a temperature greater than absolute zero emits thermal radiation. When the temperature of the body is greater than absolute zero, interatomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation are the two most important processes that determine the temperature and climate of the Earth.If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.Thermal radiation is one of the fundamental mechanisms of heat transfer.