UNIT 9: CO-ORDINATION COMPOUNDS
... Coordination isomerism: It arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex . Example [Co(NH3)6][Cr(CN)6] & [Cr(NH3)6][Co(CN)6] STEREOISOMERISM: Stereo isomers have the same chemical formula and chemical bonds but they have dif ...
... Coordination isomerism: It arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex . Example [Co(NH3)6][Cr(CN)6] & [Cr(NH3)6][Co(CN)6] STEREOISOMERISM: Stereo isomers have the same chemical formula and chemical bonds but they have dif ...
Nomenclature of metal complexes
... Disclaimer on nomenclature rules: There are at least two systems (IUPAC and everyone else) and though there is general agreement on the “big” issues, sometimes the details are slightly different. I am presenting the most common system seen by ACS journals. 1. Writing formulas: write cation, then ani ...
... Disclaimer on nomenclature rules: There are at least two systems (IUPAC and everyone else) and though there is general agreement on the “big” issues, sometimes the details are slightly different. I am presenting the most common system seen by ACS journals. 1. Writing formulas: write cation, then ani ...
Transition metal complexes and their application in drugs and
... century. In 1960 an inorganic complex cisplatin was discovered, today more than 50 years, it is still one of the world’s best selling anticancer drug. Metal complexes formed with other metals like copper, gold, gallium, germanium, tin, ruthenium, iridium was shown significant antitumor activity in a ...
... century. In 1960 an inorganic complex cisplatin was discovered, today more than 50 years, it is still one of the world’s best selling anticancer drug. Metal complexes formed with other metals like copper, gold, gallium, germanium, tin, ruthenium, iridium was shown significant antitumor activity in a ...
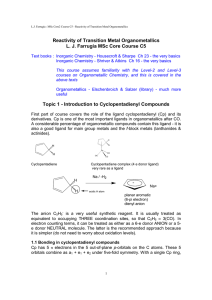
Reactivity of Transition Metal Organometallics L. J. Farrugia MSc
... These are known for the metals Ti, V, Cr , Mn, Co and Ni. Staggered or eclipsed rings leads to D5h or D5d symmetry with virtually NO barrier to rotation of the Cp ring about the metal-Cp axis. So all Cp protons appear as equivalent in the 1H ...
... These are known for the metals Ti, V, Cr , Mn, Co and Ni. Staggered or eclipsed rings leads to D5h or D5d symmetry with virtually NO barrier to rotation of the Cp ring about the metal-Cp axis. So all Cp protons appear as equivalent in the 1H ...
Co-ordination Chemistry with Macrocyclic Compounds
... for metal ions than the criptand series. This macrotricycle presents a spherical cavity, though larger than would be necessary for alkaline metal ions. It will play however an important role in supramolecular chemistry, owing to special affinity for NH 4 +, as will be shown in the following section. ...
... for metal ions than the criptand series. This macrotricycle presents a spherical cavity, though larger than would be necessary for alkaline metal ions. It will play however an important role in supramolecular chemistry, owing to special affinity for NH 4 +, as will be shown in the following section. ...
міністерство освіти і науки україни
... atom has only a few electrons in a shell, it will tend to lose them to empty the shell. These elements are metals. When metal atoms bond, a metallic bond occurs. When an atom has a nearly full electron shell, it will try to find electrons from another atom so that it can fill its outer shell. These ...
... atom has only a few electrons in a shell, it will tend to lose them to empty the shell. These elements are metals. When metal atoms bond, a metallic bond occurs. When an atom has a nearly full electron shell, it will try to find electrons from another atom so that it can fill its outer shell. These ...
Lecture note Part II (Coordination Chemistry)
... based on the octet theory of Lewis this is the first attempt to account for the bonding in complexes The formation of a complex was described as an acid base reaction according to Lewis The sum of the electrons on the central atom (Lewis acid) including those donated from the ligands (Lewis base) sh ...
... based on the octet theory of Lewis this is the first attempt to account for the bonding in complexes The formation of a complex was described as an acid base reaction according to Lewis The sum of the electrons on the central atom (Lewis acid) including those donated from the ligands (Lewis base) sh ...
Chapter 23 Metals and Metallurgy
... align opposite each other, but the spins are not equal, so there is a net magnetic field. • This can occur because magnetic centers have different numbers of unpaired electrons; more sites align in one direction than the other; both of these conditions apply. • Examples are NiMnO3, Y3Fe5O12, a ...
... align opposite each other, but the spins are not equal, so there is a net magnetic field. • This can occur because magnetic centers have different numbers of unpaired electrons; more sites align in one direction than the other; both of these conditions apply. • Examples are NiMnO3, Y3Fe5O12, a ...
Supramolecular Assemblies Built from Lanthanide
... interaction with CBs has been investigated;6 further, in their zwitterionic form, they possess a carboxylate group which can be used as a metal complexing site. This heterofunctionality has been used to build chiral one-dimensional assemblies in which lanthanide ions are bound to both L-cysteine and ...
... interaction with CBs has been investigated;6 further, in their zwitterionic form, they possess a carboxylate group which can be used as a metal complexing site. This heterofunctionality has been used to build chiral one-dimensional assemblies in which lanthanide ions are bound to both L-cysteine and ...
Triruthenium and triosmium carbonyl clusters containing chiral
... Oviedo Analytical Service. Positive FAB-MS were obtained from the University of Santiago de Compostela Mass Spectrometric Service; the m/z value given for a molecular ion corresponds to that of the most abundant isotopomer. Reaction of [Ru3 (CO)12 ] with [Hlvms]Cl. A mixture of [Ru3 (CO)12 ] (100 mg ...
... Oviedo Analytical Service. Positive FAB-MS were obtained from the University of Santiago de Compostela Mass Spectrometric Service; the m/z value given for a molecular ion corresponds to that of the most abundant isotopomer. Reaction of [Ru3 (CO)12 ] with [Hlvms]Cl. A mixture of [Ru3 (CO)12 ] (100 mg ...
Chapter 24 Chemistry of Coordination Compounds
... • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
... • As is the case with ionic compounds, the name of the cation appears first; the anion is named last. • Ligands are listed alphabetically before the metal. Prefixes denoting the number of a particular ligand are ignored when alphabetizing. ...
Old Clusters with New Function: Oxidation Catalysis by High
... redox activity than the Mn4+ ions or even the Mn3+ ions of type Mn2. We consequently presumed that the Mn3+ ions of type Mn3 are those primarily responsible for the catalytic activity of the Mn12 clusters. In order to test this assumption, we explored two molecular Mn clusters that contain only Mn3+ ...
... redox activity than the Mn4+ ions or even the Mn3+ ions of type Mn2. We consequently presumed that the Mn3+ ions of type Mn3 are those primarily responsible for the catalytic activity of the Mn12 clusters. In order to test this assumption, we explored two molecular Mn clusters that contain only Mn3+ ...
112 Exam III Lec Outline 2015
... The colors associated with compounds provide insights into their structure and bonding. Transition metals display some of the most vibrant colors, this is due to their bonding Transition metals are capable of forming highly colorized ”complex ions”, [Fe(H2O)6]3+, for example. These compounds are cal ...
... The colors associated with compounds provide insights into their structure and bonding. Transition metals display some of the most vibrant colors, this is due to their bonding Transition metals are capable of forming highly colorized ”complex ions”, [Fe(H2O)6]3+, for example. These compounds are cal ...
Syllabus - Chemistry
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
Chemistry - University of Kashmir
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
... Valence bond theory- Energy changes taking place during the formation of diatomic molecules; factors affecting the combined wave function. Bent's rule and energetics of hybridization.Resonance: Conditions, Resonance energy and examples of some inorganic molecules/ions. Molecular orbital theory- Vari ...
Review of Basic Principles: The following was adapted from The
... compared to the sp 3 hybrid of the methyl group. The remaining shortening of 0.32 Å is still substantial. We now need to confirm that it really is the π* orbital of CO that is involved in the back bonding. To do this we turn to IR (infrared) spectroscopy. If CO were bound to the metal by its carbon ...
... compared to the sp 3 hybrid of the methyl group. The remaining shortening of 0.32 Å is still substantial. We now need to confirm that it really is the π* orbital of CO that is involved in the back bonding. To do this we turn to IR (infrared) spectroscopy. If CO were bound to the metal by its carbon ...
On the Convergence of Atomic Charges with the Size of the
... for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid dissociation (pKa) can be related to the charge of the conjugate base2-6. Further, atomic charges ca ...
... for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid dissociation (pKa) can be related to the charge of the conjugate base2-6. Further, atomic charges ca ...
A Chapter 3
... that these ligands are arranged in space They have the same atom to atom bonding sequence but the atoms differ in their arrangement in space They have the same atoms, same sets of bonds but differ in the relative orientation of these bonds ...
... that these ligands are arranged in space They have the same atom to atom bonding sequence but the atoms differ in their arrangement in space They have the same atoms, same sets of bonds but differ in the relative orientation of these bonds ...
Metal Complexes and Isomerism 197. What is a coordination
... 221. Show that for the systems d1, d2, d3, d8, d9 and d10 two different spin states (high spin and low spin) are not possible. Show that for the systems d4-d7 two different spin states are possible dependent on the field strength of the ligand. 222. What type of electronic configurations for metal c ...
... 221. Show that for the systems d1, d2, d3, d8, d9 and d10 two different spin states (high spin and low spin) are not possible. Show that for the systems d4-d7 two different spin states are possible dependent on the field strength of the ligand. 222. What type of electronic configurations for metal c ...
PARAMAGNETIC RESONANCE OF FREE ATOMS OF THE ALKALI
... tions in benzene, and the narrowness of the lines agree with the assumption that the atoms are stabilized in a c:;:ystalline phase. The crystal structure of benzene has the face centered unit cell !>tca. At -195 C the dimen sions of the cell are as follows: a = 7.277 A, b = 9.452 A, and c = 6.728 A ...
... tions in benzene, and the narrowness of the lines agree with the assumption that the atoms are stabilized in a c:;:ystalline phase. The crystal structure of benzene has the face centered unit cell !>tca. At -195 C the dimen sions of the cell are as follows: a = 7.277 A, b = 9.452 A, and c = 6.728 A ...
COMPLEXES WITH BIOLOGICALLY ACTIVE LIGANDS
... quinoline nitrogen has the value-0.139, the.oxygen has-0.644 and the chlorine-0.464, respectively, whereas the nitrogen of the phosphazene ring, although having the electronic density in the range of-1.55 1.58, due to steric constraints is probably less available for interaction with the metal ions. ...
... quinoline nitrogen has the value-0.139, the.oxygen has-0.644 and the chlorine-0.464, respectively, whereas the nitrogen of the phosphazene ring, although having the electronic density in the range of-1.55 1.58, due to steric constraints is probably less available for interaction with the metal ions. ...
Perspective and prospects for pincer ligand chemistry
... or sulfonimides under mild conditions. These catalysts also transfer allyl from trifluoroallylborates to sulfonimine substrates. In addition, the palladium pincer complexes also serve as catalysts for the synthesis of allylboronic acids, allyltrifluoroborates, and allylstannanes, all of which are us ...
... or sulfonimides under mild conditions. These catalysts also transfer allyl from trifluoroallylborates to sulfonimine substrates. In addition, the palladium pincer complexes also serve as catalysts for the synthesis of allylboronic acids, allyltrifluoroborates, and allylstannanes, all of which are us ...
Oxygen Dissociation by Concerted Action of Di
... analogies with the cofactors of non-heme enzymes. We synthesize a two-dimensional array of chemically active di-iron sites on a Cu(001) surface where molecular oxygen readily dissociates at room temperature. We provide an atomic-level structural and electronic characterization before and after react ...
... analogies with the cofactors of non-heme enzymes. We synthesize a two-dimensional array of chemically active di-iron sites on a Cu(001) surface where molecular oxygen readily dissociates at room temperature. We provide an atomic-level structural and electronic characterization before and after react ...
112 Exam III Lec Outline 2016
... The colors associated with compounds provide insights into their structure and bonding. Transition metals display some of the most vibrant colors, this is due to their bonding Transition metals are capable of forming highly colorized ”complex ions”, [Fe(H2O)6]3+, for example. These compounds are cal ...
... The colors associated with compounds provide insights into their structure and bonding. Transition metals display some of the most vibrant colors, this is due to their bonding Transition metals are capable of forming highly colorized ”complex ions”, [Fe(H2O)6]3+, for example. These compounds are cal ...