Chapter 11 Chemical Reactions
... Normally, a cmpd composed of only C, H, (and maybe O) is reacted with oxygen – called “burning” Complete combustion, products are CO2 and H2O If incomplete, products are CO (or possibly just C) and H2O ...
... Normally, a cmpd composed of only C, H, (and maybe O) is reacted with oxygen – called “burning” Complete combustion, products are CO2 and H2O If incomplete, products are CO (or possibly just C) and H2O ...
full text pdf
... pollution; air pollution; manmade disasters; and natural disasters. They were concerned about the environment and 96% considered that its protection was important. In December 2012 the web site ukdebate.co.uk asked the following question of the UK public: ‘The environment is a political hot potato a ...
... pollution; air pollution; manmade disasters; and natural disasters. They were concerned about the environment and 96% considered that its protection was important. In December 2012 the web site ukdebate.co.uk asked the following question of the UK public: ‘The environment is a political hot potato a ...
June 01, 2008
... Some questions have more than one answer and some may have no answer. Each question a) to f) is worth one mark. The mark will be assigned only if the complete answer is given. Which of the above A to L: ...
... Some questions have more than one answer and some may have no answer. Each question a) to f) is worth one mark. The mark will be assigned only if the complete answer is given. Which of the above A to L: ...
Environment: The Science Behind the Stories, 4e (Withgott)
... universe is increasing, as energy is converted from high to low quality. Organisms must consume energy to maintain structure and keep entropy at bay. Low quality energy from organisms is usually released into the environment as heat. For example, if you had a bowl of oatmeal for breakfast, your dige ...
... universe is increasing, as energy is converted from high to low quality. Organisms must consume energy to maintain structure and keep entropy at bay. Low quality energy from organisms is usually released into the environment as heat. For example, if you had a bowl of oatmeal for breakfast, your dige ...
FINAL EXAM REVIEW PROBLEMS
... For each of the following reactions, predict the direction the equilibrium will shift when the volume of the container is increased. a. H2 (g) + F2 (g) 2 HF (g) b. CO (g) + 2 H2 (g) CH3OH (g) c. 2 SO3 (g) 2 SO2 (g) + O2 (g) 57. For the reaction: 2 SO2 (g) + O2 (g) 2 SO3 (g) a. If the reactio ...
... For each of the following reactions, predict the direction the equilibrium will shift when the volume of the container is increased. a. H2 (g) + F2 (g) 2 HF (g) b. CO (g) + 2 H2 (g) CH3OH (g) c. 2 SO3 (g) 2 SO2 (g) + O2 (g) 57. For the reaction: 2 SO2 (g) + O2 (g) 2 SO3 (g) a. If the reactio ...
chapter
... • About 70% of our total body weight is water • Water (via photosynthesis) is the source of oxygen in air, and hydrogen atoms used in many organic compounds • Water is a solvent for biological reactions, and a reactant or product in many chemical reactions • Water is a principal environmental factor ...
... • About 70% of our total body weight is water • Water (via photosynthesis) is the source of oxygen in air, and hydrogen atoms used in many organic compounds • Water is a solvent for biological reactions, and a reactant or product in many chemical reactions • Water is a principal environmental factor ...
PERIYAR UNIVERSITY SALEM – 636 011. PERIYAR INSTITUTE OF DISTANCE EDUCATION
... Candidates who obtain 75% of the marks in the aggregate shall be deemed to have passed the examination in First Class with Distinction provided they pass all the examinations prescribed of the course at the first appearance. Candidates who pass all the examinations prescribed for the course in the ...
... Candidates who obtain 75% of the marks in the aggregate shall be deemed to have passed the examination in First Class with Distinction provided they pass all the examinations prescribed of the course at the first appearance. Candidates who pass all the examinations prescribed for the course in the ...
Unit 8 Packet
... 2. Propane, C3H8, burns in air to form carbon dioxide and water. If 12 moles of carbon dioxide are formed, how many moles of propane were burned? Equation: Before: Change After 3. Ammonia, NH3, for fertilizer is made by causing hydrogen and nitrogen to react at high temperature and pressure. How man ...
... 2. Propane, C3H8, burns in air to form carbon dioxide and water. If 12 moles of carbon dioxide are formed, how many moles of propane were burned? Equation: Before: Change After 3. Ammonia, NH3, for fertilizer is made by causing hydrogen and nitrogen to react at high temperature and pressure. How man ...
Microbial Biogeochemistry
... • Sulfate reducers: use sulfate, SO42- + e- S or H2S, to oxidize organic compounds produced by fermentors. (chemoorganoheterotrophs). • Many genera of bacteria. Example, Desulfovibrio sp. • Phototrophic bacteria: Use light and H2S as electron donor (PS I) ...
... • Sulfate reducers: use sulfate, SO42- + e- S or H2S, to oxidize organic compounds produced by fermentors. (chemoorganoheterotrophs). • Many genera of bacteria. Example, Desulfovibrio sp. • Phototrophic bacteria: Use light and H2S as electron donor (PS I) ...
Improved Water and Land Management in the Ethiopian Highlands
... The concept of transboundary natural resources management is strongly related to ‗bioregionalism‘, which views the world as consisting of contiguous but discrete ‗bioregions‘ with the boundaries of each bioregion defined by nature rather than legislation or political expedience (cf. Wolmer 2003). Ac ...
... The concept of transboundary natural resources management is strongly related to ‗bioregionalism‘, which views the world as consisting of contiguous but discrete ‗bioregions‘ with the boundaries of each bioregion defined by nature rather than legislation or political expedience (cf. Wolmer 2003). Ac ...
AP Chemistry Summer Assignment Summer 2015 Ms. Osquist
... The problems below are organized to correspond with the chapters of your textbook. Before completing the problems, you should either read your book (if you have purchased it) or read the notes for the chapter on the ChemWiki site linked above. Complete the problems below on a separate piece of paper ...
... The problems below are organized to correspond with the chapters of your textbook. Before completing the problems, you should either read your book (if you have purchased it) or read the notes for the chapter on the ChemWiki site linked above. Complete the problems below on a separate piece of paper ...
Cycles of Matter - Brookwood High School
... warm air will cool the higher it rises which causes water vapor to condense and forms clouds ...
... warm air will cool the higher it rises which causes water vapor to condense and forms clouds ...
HIBBING COMMUNITY COLLEGE
... 47. assign oxidation numbers and identify oxidation and reduction processes. 48. describe endothermic and exothermic processes. 49. describe what happens when heat is added to a substance. 50. calculate the heat needed for phase changes and temperature changes. 51. perform calculations in calorimetr ...
... 47. assign oxidation numbers and identify oxidation and reduction processes. 48. describe endothermic and exothermic processes. 49. describe what happens when heat is added to a substance. 50. calculate the heat needed for phase changes and temperature changes. 51. perform calculations in calorimetr ...
Practice Toxins Mid-Unit Test 08-09
... ______1. What type of reaction is this? Ag (s) + CuI2 (aq) AgI (s) + Cu(s) (A) single displacement (B) double displacement (C) combination reaction (D) decomposition reaction ______2.Calcium Chloride is abbreviated (A) CaCl (C) Ca2Cl (B) CaCl2 (D) Cl2Ca ______3. What is the molarity of 3.5 moles o ...
... ______1. What type of reaction is this? Ag (s) + CuI2 (aq) AgI (s) + Cu(s) (A) single displacement (B) double displacement (C) combination reaction (D) decomposition reaction ______2.Calcium Chloride is abbreviated (A) CaCl (C) Ca2Cl (B) CaCl2 (D) Cl2Ca ______3. What is the molarity of 3.5 moles o ...
Elements (NonMetals)
... C found in CO2 of atmosphere all plants and animals contain Why are living organisms based on carbon molecules and not some other element to form backbone of complex biochemical molecules? Carbon atoms have ability to form 4 bonds to form long chains or rings of like atoms and have other atoms attac ...
... C found in CO2 of atmosphere all plants and animals contain Why are living organisms based on carbon molecules and not some other element to form backbone of complex biochemical molecules? Carbon atoms have ability to form 4 bonds to form long chains or rings of like atoms and have other atoms attac ...
Impact of fertilizers on aquatic ecosystems and protection of water
... these problems are fertilizers. Industrial production and the usage of fertilizers have led to a sharp increase in food production that has been accompanied by the population growth in almost all countries around the world. While fertilizers restored into soil have had the requested affect, consider ...
... these problems are fertilizers. Industrial production and the usage of fertilizers have led to a sharp increase in food production that has been accompanied by the population growth in almost all countries around the world. While fertilizers restored into soil have had the requested affect, consider ...
CHEM1100 Practice Exam 2 You have 120 minutes to complete this
... 14. Consider the following chemical reaction: 3H2 + N2 → 2NH3 If 3.30 mol of H2 react with excess nitrogen (N2) how many grams of ammonia (NH3) should be produced? Suppose the actual amount of nitrogen produced is 21.50 g. Calculate the reaction yield. ...
... 14. Consider the following chemical reaction: 3H2 + N2 → 2NH3 If 3.30 mol of H2 react with excess nitrogen (N2) how many grams of ammonia (NH3) should be produced? Suppose the actual amount of nitrogen produced is 21.50 g. Calculate the reaction yield. ...
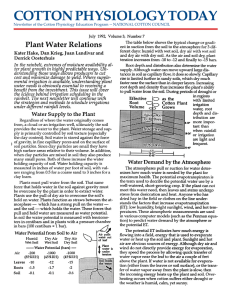
Plant Water Relations - National Cotton Council
... soils with high pH, redUCing (water logged) conditions for 24 hours can decrease the aVailability of iron, resulting in symptoms of iron deficiency throughout the plant. Iron deficiency looks similar to nitrogen deficiency but occurs more rapidly after flooding and only in high pH soil. Upper leaves ...
... soils with high pH, redUCing (water logged) conditions for 24 hours can decrease the aVailability of iron, resulting in symptoms of iron deficiency throughout the plant. Iron deficiency looks similar to nitrogen deficiency but occurs more rapidly after flooding and only in high pH soil. Upper leaves ...