Understanding the Role of Aqueous Solution in Chemical Reactions
... Water is a unique solvent due its structure and reactivity. The ability of a water molecule to form hydrogen bonds is its most important characteristic. In liquid water, each molecule typically donates and accepts two hydrogen bonds. This structure significantly enhances water’s ability to accept or ...
... Water is a unique solvent due its structure and reactivity. The ability of a water molecule to form hydrogen bonds is its most important characteristic. In liquid water, each molecule typically donates and accepts two hydrogen bonds. This structure significantly enhances water’s ability to accept or ...
Stoichiometry/Mass/Mole Relationships
... 10. ___ C6H12 + ___ O2 → ___ CO2 + ___ H2O 42 grams of cyclohexane burns in excess air to from carbon dioxide and water. How many grams of carbon dioxide and of water vapor are produced? ...
... 10. ___ C6H12 + ___ O2 → ___ CO2 + ___ H2O 42 grams of cyclohexane burns in excess air to from carbon dioxide and water. How many grams of carbon dioxide and of water vapor are produced? ...
Spatial distribution of heavy metal contamination in surface
... planet: it is the 21st-longest river, it has the 25thlargest river basin (817,000 km2) [1] and it is the second longest river in Europe, flowing along 2857 km from Germany’s Black Forest to its delta on the Black Sea [2]. Heavy metals in the environment originate from various sources: industrial act ...
... planet: it is the 21st-longest river, it has the 25thlargest river basin (817,000 km2) [1] and it is the second longest river in Europe, flowing along 2857 km from Germany’s Black Forest to its delta on the Black Sea [2]. Heavy metals in the environment originate from various sources: industrial act ...
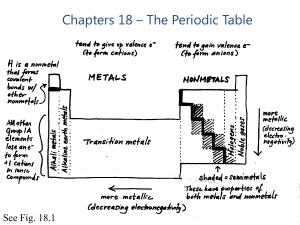
Chapters 18 – The Periodic Table
... Common behavior: reaction with metal to become 2- ion in ionic compound; for most metals, most common minerals are oxides or sulfides Covalent bonds with other NMs; series of covalent hydrides (H2X) All but O have d orbitals available, so more than an octet is common Te and Po can be 4+ cati ...
... Common behavior: reaction with metal to become 2- ion in ionic compound; for most metals, most common minerals are oxides or sulfides Covalent bonds with other NMs; series of covalent hydrides (H2X) All but O have d orbitals available, so more than an octet is common Te and Po can be 4+ cati ...
Role of Chemistry in Everyday Life
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...
Chemical Reactions and Stoichiometry
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
Functional Groups
... Organic carbon: Think waters overrun by septic systems… If these effluents contain nutrients (N, K, P) which are often limited in the natural environment, it can lead to eutrophication. BOD: (biological oxygen demand) the capacity of the organic matter in a sample of natural water to consume oxygen. ...
... Organic carbon: Think waters overrun by septic systems… If these effluents contain nutrients (N, K, P) which are often limited in the natural environment, it can lead to eutrophication. BOD: (biological oxygen demand) the capacity of the organic matter in a sample of natural water to consume oxygen. ...
Are You suprised ?
... pressure of 0.329 atm at 35oC. At this temperature, the vapor pressure of pure acetone is 0.453 atm, and the vapor pressure of pure chloroform is 0.388 atm. By comparing the measured vapor pressure and the calculated one, the above solution is: A) Endothermic solution ...
... pressure of 0.329 atm at 35oC. At this temperature, the vapor pressure of pure acetone is 0.453 atm, and the vapor pressure of pure chloroform is 0.388 atm. By comparing the measured vapor pressure and the calculated one, the above solution is: A) Endothermic solution ...
Summer_Assignment_AP_Chemistry_TW 2015
... Do all of your homework assignments. Some teachers look simply for effort. Practice makes perfect test scores. By doing problems related to your current material, you can be fully readied for that material's test or quiz. ...
... Do all of your homework assignments. Some teachers look simply for effort. Practice makes perfect test scores. By doing problems related to your current material, you can be fully readied for that material's test or quiz. ...
7.5.9 Compare physical properties of matter to the chemical property
... Density is a property that describes the relationship between the mass of a material and its volume Substances that are denser contain MORE matter in a given volume ...
... Density is a property that describes the relationship between the mass of a material and its volume Substances that are denser contain MORE matter in a given volume ...
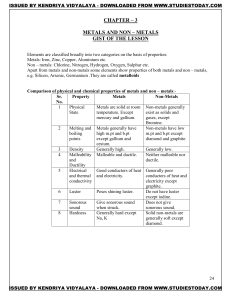
METALS AND NON – METALS Concepts
... Elements are classified broadly into two categories on the basis of properties: Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Ar ...
... Elements are classified broadly into two categories on the basis of properties: Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Ar ...
CHAPTER 1 Chemical Foundations
... 26. The combination reaction that occurs between lithium metal and fluorine gas. ...
... 26. The combination reaction that occurs between lithium metal and fluorine gas. ...
Free response review
... c. The radius of an oxide ion is larger than the radius of an oxygen atom. d. The first ionization energy of aluminum is smaller than the first ionization energy of magnesium e. The third ionization energy of an element is always larger than its second ionization energy 2. Write the formulas to show ...
... c. The radius of an oxide ion is larger than the radius of an oxygen atom. d. The first ionization energy of aluminum is smaller than the first ionization energy of magnesium e. The third ionization energy of an element is always larger than its second ionization energy 2. Write the formulas to show ...
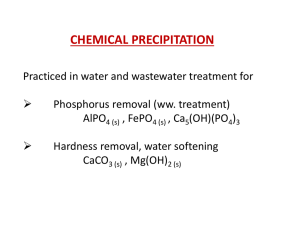
Phosphorus Removal from Wastewater by Chemical Precipitation
... • There are a variety of chemical application points in the treatment scheme. • The common addition points for metals in biological treatment plants are before the primary clarifier or before the secondary clarifier or during tertiary treatment. • Some alternative treatment schemes are shown in the ...
... • There are a variety of chemical application points in the treatment scheme. • The common addition points for metals in biological treatment plants are before the primary clarifier or before the secondary clarifier or during tertiary treatment. • Some alternative treatment schemes are shown in the ...
File - Flipped Out Science with Mrs. Thomas!
... coefficients mean? How can you tell which elements are present. Know the Law of Conservation of Mass and how it is applied to chemical reactions Know what makes a compound organic or not Know the difference between physical and chemical properties and changes Exothermic and endothermic react ...
... coefficients mean? How can you tell which elements are present. Know the Law of Conservation of Mass and how it is applied to chemical reactions Know what makes a compound organic or not Know the difference between physical and chemical properties and changes Exothermic and endothermic react ...
Environmental Chemistry
... consume oxygen, a process usually catalyzed by bacteria, is called BOD Procedure: measure O2 in the stream or lake. Take a sample and store at 25oC for five days and remeasure O2 content. The difference is the BOD – BOD5 corresponds to about 80% of the actual value. It is not practical to measure th ...
... consume oxygen, a process usually catalyzed by bacteria, is called BOD Procedure: measure O2 in the stream or lake. Take a sample and store at 25oC for five days and remeasure O2 content. The difference is the BOD – BOD5 corresponds to about 80% of the actual value. It is not practical to measure th ...
File
... 19. What are the respective concentrations (M) of Fe3+ and I- afforded by dissolving 0.200 mol FeI3 in water and diluting to 725 mL? A) 0.276 and 0.828 B) 0.828 and 0.276 C) 0.276 and 0.276 D) 0.145 and 0.435 20. When solutions of Ca(NO3)2 and Na3PO4 are mixed, they form a precipitate of Ca3(PO4)2. ...
... 19. What are the respective concentrations (M) of Fe3+ and I- afforded by dissolving 0.200 mol FeI3 in water and diluting to 725 mL? A) 0.276 and 0.828 B) 0.828 and 0.276 C) 0.276 and 0.276 D) 0.145 and 0.435 20. When solutions of Ca(NO3)2 and Na3PO4 are mixed, they form a precipitate of Ca3(PO4)2. ...
Earth Systems Review
... What is an estuary and how can aqueducts impact an estuarine environment? Estuaries are formed where salt water meets fresh water. Aqueducts remove fresh water from a river and thus make the estuary more saline which reduces the species richness of the estuary. ...
... What is an estuary and how can aqueducts impact an estuarine environment? Estuaries are formed where salt water meets fresh water. Aqueducts remove fresh water from a river and thus make the estuary more saline which reduces the species richness of the estuary. ...
paper 2 revision booklet
... District Here the buildings were wooden and not securely attached to their foundations. The area was also located on an old landfill site, which was created following the devastation caused by the 1906 earthquake. As a result of the weak sub-surface sediments, when the ground shook in the 1989 earth ...
... District Here the buildings were wooden and not securely attached to their foundations. The area was also located on an old landfill site, which was created following the devastation caused by the 1906 earthquake. As a result of the weak sub-surface sediments, when the ground shook in the 1989 earth ...
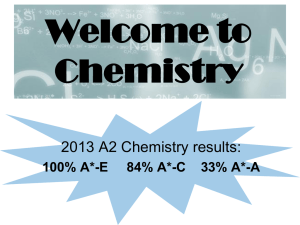
Welcome to Chemistry
... - Amounts of substance e.g. molecular formula, empirical formula, reacting mass ...
... - Amounts of substance e.g. molecular formula, empirical formula, reacting mass ...