2. Water Security in Coastal Region of BangladeshBEJS 9.2 Final
... Association, up to 2008 there were 13,080 desalination plants all over the world producing more than 12 billion gallons of water per day (www.idadesal.org). The intense water crisis all over the world due to population growth, wasteful use of water, pollution, diversion of international river flow a ...
... Association, up to 2008 there were 13,080 desalination plants all over the world producing more than 12 billion gallons of water per day (www.idadesal.org). The intense water crisis all over the world due to population growth, wasteful use of water, pollution, diversion of international river flow a ...
Physical and Chemical Change
... substances are formed during a chemical reaction. When matter undergoes a change, it is classified as either a physical change or chemical change. During a physical change, only the size, temperature or physical state of the substance changes. Melting, dissolving, grinding and evaporating are all ph ...
... substances are formed during a chemical reaction. When matter undergoes a change, it is classified as either a physical change or chemical change. During a physical change, only the size, temperature or physical state of the substance changes. Melting, dissolving, grinding and evaporating are all ph ...
Environmental Information System for Uzbekistan
... from EIDB were determined, created and inputted; The module for creation of thematic maps according to indicators were developed. ...
... from EIDB were determined, created and inputted; The module for creation of thematic maps according to indicators were developed. ...
Flexbook - What is Matter?
... The formula for a compound uses the symbols to indicate the type of atoms involved and uses subscripts to indicate the number of each atom in the formula. For example, aluminum combines with oxygen to form the compound aluminum oxide. Forming aluminum oxide requires two atoms of aluminum and three a ...
... The formula for a compound uses the symbols to indicate the type of atoms involved and uses subscripts to indicate the number of each atom in the formula. For example, aluminum combines with oxygen to form the compound aluminum oxide. Forming aluminum oxide requires two atoms of aluminum and three a ...
ROOT WATERING SYSTEM: PROVeN ResulTs
... and researcher, Dr. Ursula Schuch. The research included 48 ash trees at a test site. Half of the trees were irrigated with a pair of Rain Bird® RWS-M 18" basket weave canisters that were installed in-ground near the root ball. The other half was surface irrigated with bubblers near the tree stem. A ...
... and researcher, Dr. Ursula Schuch. The research included 48 ash trees at a test site. Half of the trees were irrigated with a pair of Rain Bird® RWS-M 18" basket weave canisters that were installed in-ground near the root ball. The other half was surface irrigated with bubblers near the tree stem. A ...
physical and chemical change
... chemical changes in matter. Background: Matter has both physical and chemical properties. A physical property is a quality or condition of a substance that can be observed or measured without changing the composition of the substance. Color is an example of a physical property. During a physical cha ...
... chemical changes in matter. Background: Matter has both physical and chemical properties. A physical property is a quality or condition of a substance that can be observed or measured without changing the composition of the substance. Color is an example of a physical property. During a physical cha ...
Physical Geography
... importance of land and water forms and related processes to economic activity. For example, farming became established in certain areas due to the presence of volcanic soils; tourism resulted from distinctive features created by moving ice; the life cycle of a given river led to the establishment of ...
... importance of land and water forms and related processes to economic activity. For example, farming became established in certain areas due to the presence of volcanic soils; tourism resulted from distinctive features created by moving ice; the life cycle of a given river led to the establishment of ...
Ch 4 Types of Chemical Reactions and Solution Stoichiometry
... What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M sulfuric acid solution? (answer = 9.4 mL) (16 M)(x L) = (0.10 M)(1.5 L) x = 0.0094 L or 9.4 mL ...
... What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M sulfuric acid solution? (answer = 9.4 mL) (16 M)(x L) = (0.10 M)(1.5 L) x = 0.0094 L or 9.4 mL ...
Types of Chemical Reactions
... Types of decomposition reactions – Decomposition of Binary Compounds – Decomposition of Metal Carbonates – Decomposition of Metal Hydroxides – Decomposition of Metal Chlorates – Decomposition of Acids ...
... Types of decomposition reactions – Decomposition of Binary Compounds – Decomposition of Metal Carbonates – Decomposition of Metal Hydroxides – Decomposition of Metal Chlorates – Decomposition of Acids ...
Chapter 1 Matter and Change
... States of matter 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a l ...
... States of matter 1) Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. – Vapor- a substance that is currently a gas, but normally is a l ...
+ H 2 O(l )
... Product of strong acid + strong base • When a strong acid and a strong base react the chemical change that always occurs is that H+ ions react with OHto form water. • Strong acid- HCl, HNO3, H2SO4 • Strong base-NaOH, KOH ...
... Product of strong acid + strong base • When a strong acid and a strong base react the chemical change that always occurs is that H+ ions react with OHto form water. • Strong acid- HCl, HNO3, H2SO4 • Strong base-NaOH, KOH ...
Test
... 79 Explain, using LeChatelier’s principle, why decreasing the concentration of CO2 decreases the concentration of H2CO3. [1] 80 What is the oxidation number of carbon in H2CO3(aq)? ...
... 79 Explain, using LeChatelier’s principle, why decreasing the concentration of CO2 decreases the concentration of H2CO3. [1] 80 What is the oxidation number of carbon in H2CO3(aq)? ...
The Origin of the Natural Water Chemical Composition in the
... processes in the eastern slope of the Polar Ural where permafrost is widely spread. To date, this region has not been studied in detail. However, it is very important to have information on the natural waters in this region because they play a significant role in all geochemical processes, including ...
... processes in the eastern slope of the Polar Ural where permafrost is widely spread. To date, this region has not been studied in detail. However, it is very important to have information on the natural waters in this region because they play a significant role in all geochemical processes, including ...
Types of Reactions and Solution Chemistry
... This overall reaction is the molecular equation. But what is really going on in this aqueous state? Notice, we have formed a solid. That means that ions that were once dissolved in solution came together and made a solid, or a precipitate. The ionic equation will help us determine what is going on i ...
... This overall reaction is the molecular equation. But what is really going on in this aqueous state? Notice, we have formed a solid. That means that ions that were once dissolved in solution came together and made a solid, or a precipitate. The ionic equation will help us determine what is going on i ...
AP Chemistry Summer Assignment 2016
... test on Chapters 3 and 4 during the first few days of school In addition to the actual summer assignment there is one unit pre-assignment (Chapter 5, Gases) that will not be due until just before we start this actual unit during the school year. If you have a demanding schedule this fall or struggle ...
... test on Chapters 3 and 4 during the first few days of school In addition to the actual summer assignment there is one unit pre-assignment (Chapter 5, Gases) that will not be due until just before we start this actual unit during the school year. If you have a demanding schedule this fall or struggle ...
04_3eTIF
... 1) What is bioremediation? How can it help with environmental problems? Give examples and list some advantages and disadvantages to its use. Answer: Bioremediation is a process in which organisms are used to metabolize toxins to remove them from the environment. In this process, the natural processe ...
... 1) What is bioremediation? How can it help with environmental problems? Give examples and list some advantages and disadvantages to its use. Answer: Bioremediation is a process in which organisms are used to metabolize toxins to remove them from the environment. In this process, the natural processe ...
Unit 7 Packet
... Hydrazine (N2H4) and hydrogen peroxide are used together as rocket fuel. The products are nitrogen gas and water. ...
... Hydrazine (N2H4) and hydrogen peroxide are used together as rocket fuel. The products are nitrogen gas and water. ...
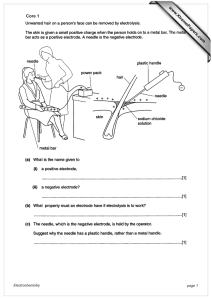
Core 1 www.XtremePapers.com Electrochemistry page 1
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
Chapter 8
... – The formulas of the reactants and products must be correct. – The reactants are written to the left of the arrow and the products to the right of the arrow. ...
... – The formulas of the reactants and products must be correct. – The reactants are written to the left of the arrow and the products to the right of the arrow. ...
Jay Shri Harsiddhi Mataji Pick the date
... Porous article is covered with glaze to get water tight article which is done by melting it over the surface of the body. Quartz, feldspar, boric oxide are the constituent of the glaze which are finely ground and mixed with water which forms slip then it is fired to higher temperature to produce smo ...
... Porous article is covered with glaze to get water tight article which is done by melting it over the surface of the body. Quartz, feldspar, boric oxide are the constituent of the glaze which are finely ground and mixed with water which forms slip then it is fired to higher temperature to produce smo ...
Flint Water Crisis Response 4/11/2016 - M
... while vitamin D may enhance absorption and bone storage. Studies in adults have shown higher lead absorption rates when fasting. Mr. Burka followed up, describing preliminary conversation with DHHS regarding health assessment strategies. Specifically, Medicaid will reimburse fully for vitamins that ...
... while vitamin D may enhance absorption and bone storage. Studies in adults have shown higher lead absorption rates when fasting. Mr. Burka followed up, describing preliminary conversation with DHHS regarding health assessment strategies. Specifically, Medicaid will reimburse fully for vitamins that ...
Organic Compound Synthesis on the Primitive Earth
... Synthesis of Organic Compounds At the present time the direct or indirect source of free energy for all living organisms is the sunlight utilized by photosynthetic organisms. But before the evolution of photosynthesis other sources of free energy must have been used. It is of interest to consider th ...
... Synthesis of Organic Compounds At the present time the direct or indirect source of free energy for all living organisms is the sunlight utilized by photosynthetic organisms. But before the evolution of photosynthesis other sources of free energy must have been used. It is of interest to consider th ...
rocks and minerals quiz
... Example 1. Determine if the following substances are: pure elements, pure compounds, homogeneous mixtures, or heterogeneous mixtures. 1a. argon (g) A. Argon is atomic number 18 on the periodic table, which makes it an atom. Argon is a pure element. 1b. table sugar (s) A. Table sugar is composed sole ...
... Example 1. Determine if the following substances are: pure elements, pure compounds, homogeneous mixtures, or heterogeneous mixtures. 1a. argon (g) A. Argon is atomic number 18 on the periodic table, which makes it an atom. Argon is a pure element. 1b. table sugar (s) A. Table sugar is composed sole ...
Amounts of Reactants and Products
... 5. Convert from moles back to grams if required by the problem. Sample Problems: a) Solid lithium hydroxide (LiOH) is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate (Li2CO3) and liquid water. What mass of gaseous carbon dioxide ...
... 5. Convert from moles back to grams if required by the problem. Sample Problems: a) Solid lithium hydroxide (LiOH) is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate (Li2CO3) and liquid water. What mass of gaseous carbon dioxide ...