Higher Chemistry summary 3a
... Therefore, for every 0.5 moles of methane 1 mole of oxygen would be required. Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen wil ...
... Therefore, for every 0.5 moles of methane 1 mole of oxygen would be required. Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen wil ...
full text - pdf 452 kB
... The AH and AC' values for reactions (6) and (7) are shown in Fig. 5 (1 1, 14). The AH values do not change significantly with temperature and the AC, values are small for reactions (6) and (7). The AC,, AH, and AS values for the association of K+, Ba2+, and Sr2+ with 18-crown-6 (18C6) are large and ...
... The AH and AC' values for reactions (6) and (7) are shown in Fig. 5 (1 1, 14). The AH values do not change significantly with temperature and the AC, values are small for reactions (6) and (7). The AC,, AH, and AS values for the association of K+, Ba2+, and Sr2+ with 18-crown-6 (18C6) are large and ...
Acid and Bases: Alkalinity and pH in Natural Waters.
... At the beginning of the past century (ca. 1920), chemists established the conceptual framework for dealing with acid-base reactions. They realized the important role of the proton, H+, and the fact that this proton was actually hydrated when in solution, i.e.present with a certain number of water mo ...
... At the beginning of the past century (ca. 1920), chemists established the conceptual framework for dealing with acid-base reactions. They realized the important role of the proton, H+, and the fact that this proton was actually hydrated when in solution, i.e.present with a certain number of water mo ...
Spectroscopy In Oceanography
... sea with its boundaries (continents, sea-floor and atmosphere), with its constituents (marine plants and animals), and more recently from man's direct intervention, cause compositional changes, the understanding and measuring of which give us marine chemists our jobs. The development of chemical oce ...
... sea with its boundaries (continents, sea-floor and atmosphere), with its constituents (marine plants and animals), and more recently from man's direct intervention, cause compositional changes, the understanding and measuring of which give us marine chemists our jobs. The development of chemical oce ...
Chemical bonding
... are absorbed or released during a chemical reaction. • If NRG is absorbed in the RXN, it is endothermic • If NRG is released in the RXN, it is exothermic ...
... are absorbed or released during a chemical reaction. • If NRG is absorbed in the RXN, it is endothermic • If NRG is released in the RXN, it is exothermic ...
OCR Document
... – Water dissolves and carries elements down into geosphere and groundwater. – Important part of all geochemical cycles i.e. - important for transporting nutrient elements essential for life ...
... – Water dissolves and carries elements down into geosphere and groundwater. – Important part of all geochemical cycles i.e. - important for transporting nutrient elements essential for life ...
Soil salinity
... water entitlements to be bought and sold without any attachment to land. It is hoped that this will transfer water resources to higher value uses and decrease use of water on land not suited to irrigation. TWE should result in the increased adoption of water-saving technologies, as water that can be ...
... water entitlements to be bought and sold without any attachment to land. It is hoped that this will transfer water resources to higher value uses and decrease use of water on land not suited to irrigation. TWE should result in the increased adoption of water-saving technologies, as water that can be ...
Chapters 13 and 14
... d. Carbon dioxide, rather than a stream of water, should be used to extinguish an oil fire. 9. 1987 #7a-b In 1884 the Swedish chemist Svante Arrhenius proposed that salts dissociate into two or more separate, independent, ionic fragments when they dissolve in water. a. Give one piece of experimental ...
... d. Carbon dioxide, rather than a stream of water, should be used to extinguish an oil fire. 9. 1987 #7a-b In 1884 the Swedish chemist Svante Arrhenius proposed that salts dissociate into two or more separate, independent, ionic fragments when they dissolve in water. a. Give one piece of experimental ...
Calculations with Chemical Formulas and Equations
... a. When conducting this type of experiment, you are assuming that all of the carbon and hydrogen show up in the CO2 and H2O, respectively. In this experiment, where all of the carbon and hydrogen do not show up, when you analyze the CO2 for carbon and H2O for hydrogen, you will find that the weights ...
... a. When conducting this type of experiment, you are assuming that all of the carbon and hydrogen show up in the CO2 and H2O, respectively. In this experiment, where all of the carbon and hydrogen do not show up, when you analyze the CO2 for carbon and H2O for hydrogen, you will find that the weights ...
Chemical Formulas and Chemical Compounds
... 7. The oxidation numbers assigned to the atoms in some organic compounds have unexpected values. Assign oxidation numbers to each atom in the following compounds: (Note: Some oxidation numbers may not be whole numbers.) a. CO2 Carbon is ⫹4 and each oxygen is ⫺2. b. CH4 (methane) Carbon is ⫺4 and eac ...
... 7. The oxidation numbers assigned to the atoms in some organic compounds have unexpected values. Assign oxidation numbers to each atom in the following compounds: (Note: Some oxidation numbers may not be whole numbers.) a. CO2 Carbon is ⫹4 and each oxygen is ⫺2. b. CH4 (methane) Carbon is ⫺4 and eac ...
Coherence Domain - Conference on the Physics, Chemistry and
... temperature. Thus a non aqueous molecule is attracted in the CD if a frequency ν is present in its spectrum such that ...
... temperature. Thus a non aqueous molecule is attracted in the CD if a frequency ν is present in its spectrum such that ...
identifying sources fecal coliform in TBay
... of the water quality standards because of the fact that some of the coliform bacteria had been identified as being drug resistant. This was the beginning of utilizing this information to expand and develop methods of identifying different sources of bacteria as pollutants in water. In 1978 two-facul ...
... of the water quality standards because of the fact that some of the coliform bacteria had been identified as being drug resistant. This was the beginning of utilizing this information to expand and develop methods of identifying different sources of bacteria as pollutants in water. In 1978 two-facul ...
Chemistry 20
... It is designed for Academic Upgrading placement purposes only. This test may not be used for admission to any SAIT program; that is, this is not a SAIT admission exam. In addition, the results cannot be used at any other educational institution. The time allotted for the Chemistry 20 Placement test ...
... It is designed for Academic Upgrading placement purposes only. This test may not be used for admission to any SAIT program; that is, this is not a SAIT admission exam. In addition, the results cannot be used at any other educational institution. The time allotted for the Chemistry 20 Placement test ...
Heats of Formation WS
... Heats of Formation 1. For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and use the appendix to obtain the value of ∆Hfº. [a] NO2 (g) ...
... Heats of Formation 1. For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and use the appendix to obtain the value of ∆Hfº. [a] NO2 (g) ...
Reactions and Stoichiometry Practice Problems
... 29) Aqueous aluminum chloride and water are formed when solid aluminum oxide is submersed in hydrochloric acid. Find the mass of aluminum oxide that reacts when 0.250 moles of HCl react. ...
... 29) Aqueous aluminum chloride and water are formed when solid aluminum oxide is submersed in hydrochloric acid. Find the mass of aluminum oxide that reacts when 0.250 moles of HCl react. ...
Chemistry Chapter 9.1 Making Predictions About Solubility
... treated waste water into a lake or river is a point source of pollution. Includes: wrecked tankers that leak oil, factories that discharge metallic ions, organic compounds, acids and bases. Point source water can be of thermal pollution when thermal power plants discharge warm water into a lake. Dif ...
... treated waste water into a lake or river is a point source of pollution. Includes: wrecked tankers that leak oil, factories that discharge metallic ions, organic compounds, acids and bases. Point source water can be of thermal pollution when thermal power plants discharge warm water into a lake. Dif ...
material safety data sheet
... ACCIDENTAL RELEASE MEASURES: Wipe up with absorbent towels or mop. Avoid contact with organic materials. SECTION 6 NOTES: Follow applicable OSHA regulations (29 CFR 1910.120) SECTION 7: HANDLING AND STORAGE HANDLING AND STORAGE: Avoid ingestion, skin contact, eye contact, and inhalation OTHER PRECAU ...
... ACCIDENTAL RELEASE MEASURES: Wipe up with absorbent towels or mop. Avoid contact with organic materials. SECTION 6 NOTES: Follow applicable OSHA regulations (29 CFR 1910.120) SECTION 7: HANDLING AND STORAGE HANDLING AND STORAGE: Avoid ingestion, skin contact, eye contact, and inhalation OTHER PRECAU ...
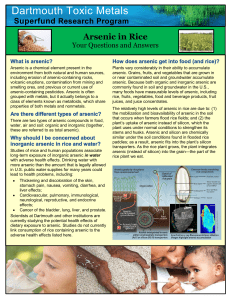
Arsenic in Rice Fact Sheet
... Plants vary considerably in their ability to accumulate arsenic. Grains, fruits, and vegetables that are grown in or near contaminated soil and groundwater accumulate arsenic. Because both organic and inorganic arsenic are commonly found in soil and groundwater in the U.S., many foods have measurabl ...
... Plants vary considerably in their ability to accumulate arsenic. Grains, fruits, and vegetables that are grown in or near contaminated soil and groundwater accumulate arsenic. Because both organic and inorganic arsenic are commonly found in soil and groundwater in the U.S., many foods have measurabl ...
Environmental Science Final Review Chapter 1 .1 • Define
... Describe three possible short-term effects and long-term effects of air pollution on human health. ...
... Describe three possible short-term effects and long-term effects of air pollution on human health. ...
Chemistry in Biology
... substrates. • The specific location where a substrate binds on an enzyme is called the active site. • The active site changes shape and forms the enzyme-substrate complex, which helps chemical bonds in the reactants to be broken and new bonds to form. ...
... substrates. • The specific location where a substrate binds on an enzyme is called the active site. • The active site changes shape and forms the enzyme-substrate complex, which helps chemical bonds in the reactants to be broken and new bonds to form. ...
Notes
... or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ice cube ...
... or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ice cube ...
Complete the following equations
... Name three major elements in the atmosphere and describe briefly their extraction process from the atmosphere. There is more oxygen (by mass) in the ocean than in the atmosphere. Why oxygen is commercially obtained from the atmosphere and not from the ocean? (Nitrogen, oxygen and argon. Gases are ex ...
... Name three major elements in the atmosphere and describe briefly their extraction process from the atmosphere. There is more oxygen (by mass) in the ocean than in the atmosphere. Why oxygen is commercially obtained from the atmosphere and not from the ocean? (Nitrogen, oxygen and argon. Gases are ex ...
Petroleum Ether
... If this product is swallowed, DO NOT INDUCE VOMITING. Give small quantities (<250 ml) of water to drink. Never give anything by mouth to an unconscious person. Seek immediate medical attention Notes to doctor/physician Aspiration of solvent may cause chemical pneumonitis. 4.2 Most important symptoms ...
... If this product is swallowed, DO NOT INDUCE VOMITING. Give small quantities (<250 ml) of water to drink. Never give anything by mouth to an unconscious person. Seek immediate medical attention Notes to doctor/physician Aspiration of solvent may cause chemical pneumonitis. 4.2 Most important symptoms ...
WELCOME TO AP CHEMISTRY
... I am very much looking forward to meeting you in September and teaching you second-year Chemistry in preparation for the AP Test in May, 2014. In September we will start out running and then proceed to a gallop as the months go by! The summer assignment is attached. The purpose of the assignment is ...
... I am very much looking forward to meeting you in September and teaching you second-year Chemistry in preparation for the AP Test in May, 2014. In September we will start out running and then proceed to a gallop as the months go by! The summer assignment is attached. The purpose of the assignment is ...