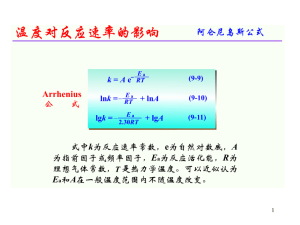
Group 2 Elements
... •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours by Group 1 and 2 compounds in terms of electron transitions •know the flame colours for Groups 1 and 2 compounds •understand experimental procedures to sh ...
... •know the reactions of the elements Mg to Ba in Group 2 with oxygen, chlorine and water •understand the formation of characteristic flame colours by Group 1 and 2 compounds in terms of electron transitions •know the flame colours for Groups 1 and 2 compounds •understand experimental procedures to sh ...
Partial melting - simple process, huge global
... Rocks are made of minerals, which all have different melting points. Minerals containing oxygen (O) and silicon (Si) have the lowest melting points, whilst minerals that contain iron (Fe) and magnesium (Mg) have the highest melting points. When rocks are heated, they often don’t melt completely – th ...
... Rocks are made of minerals, which all have different melting points. Minerals containing oxygen (O) and silicon (Si) have the lowest melting points, whilst minerals that contain iron (Fe) and magnesium (Mg) have the highest melting points. When rocks are heated, they often don’t melt completely – th ...
Characteristics of Emissions from Municipal Waste Landfills
... acidogenic, acetogenic and methanogenic bacteria. The first two stages are dominated by both obligate anaerobes (Bacillus, Pseudomonas, Clostridium, Bifidobacterium) and facultative anaerobes (Streptococcus, Enterobacterium). Some acidogenic bacteria are obligate anaerobes (Aerobacter, Alcaligenes, ...
... acidogenic, acetogenic and methanogenic bacteria. The first two stages are dominated by both obligate anaerobes (Bacillus, Pseudomonas, Clostridium, Bifidobacterium) and facultative anaerobes (Streptococcus, Enterobacterium). Some acidogenic bacteria are obligate anaerobes (Aerobacter, Alcaligenes, ...
Chemistry II Exams and Keys 2013 Season
... 10. A sample containing 7.45 grams of KCl is dissolved in sufficient distilled water and reacted with 1000.0 mL 0.300 M AgNO3 solution. Excess silver nitrate solution reacted with the metallic copper according to the following reaction: Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s) The precipitate is f ...
... 10. A sample containing 7.45 grams of KCl is dissolved in sufficient distilled water and reacted with 1000.0 mL 0.300 M AgNO3 solution. Excess silver nitrate solution reacted with the metallic copper according to the following reaction: Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s) The precipitate is f ...
1st Olympiad of Metropolises Chemistry Theoretical Problems
... formed during a sunny summer day (8 h) in Moscow. The necessary information: solar energy absorbed by Moscow region in summer time – 150 Wm–2; the Gibbs energy of photosynthesis is 480 kJ/mol of CO2; green plants absorb ~10% of the available solar energy; 25% of the absorbed energy is used ...
... formed during a sunny summer day (8 h) in Moscow. The necessary information: solar energy absorbed by Moscow region in summer time – 150 Wm–2; the Gibbs energy of photosynthesis is 480 kJ/mol of CO2; green plants absorb ~10% of the available solar energy; 25% of the absorbed energy is used ...
Unit 8 Homework Packet
... 27. One process for the commercial production of baking soda (sodium hydrogen carbonate) involves the following reaction, in which the carbon dioxide is used in its solid form ("dry ice") both to serve as a source of reactant and to cool the reaction system to a temperature low enough for the sodium ...
... 27. One process for the commercial production of baking soda (sodium hydrogen carbonate) involves the following reaction, in which the carbon dioxide is used in its solid form ("dry ice") both to serve as a source of reactant and to cool the reaction system to a temperature low enough for the sodium ...
Example 1: An experiment shows that 64g of
... 2. Write balanced equations from the information given below (wherever you can include state symbols) (s) = solid ...
... 2. Write balanced equations from the information given below (wherever you can include state symbols) (s) = solid ...
Key - GCC
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
Wipe Away Degreaser Purple Power
... Disclaimer: James Austin Company provides this information without warranty. The information is believed to be accurate, but James Austin Company makes no representations as to its accuracy. The information should be used to make an independant determination and therefore, users are responsible to v ...
... Disclaimer: James Austin Company provides this information without warranty. The information is believed to be accurate, but James Austin Company makes no representations as to its accuracy. The information should be used to make an independant determination and therefore, users are responsible to v ...
Optra S High Yield Return Program Print Cartridge for Label
... Low acute inhalation toxicity. As with exposure to high concentrations of any dust, minimal irritation of the respiratory tract may occur. Pure carbon black, a minor component of this product, has been listed by IARC as a group 2B (possible carcinogen). This classification is based on rat "lung part ...
... Low acute inhalation toxicity. As with exposure to high concentrations of any dust, minimal irritation of the respiratory tract may occur. Pure carbon black, a minor component of this product, has been listed by IARC as a group 2B (possible carcinogen). This classification is based on rat "lung part ...
Chemical Equation Reactions
... molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually contains individual IONS, not molecules, in solution. By looking at the afo ...
... molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually contains individual IONS, not molecules, in solution. By looking at the afo ...
WD-40 Low Odour
... Suitable Extinguishing Media: Use water fog, dry chemical, carbon dioxide or foam. Do not use water jet or flooding amounts of water. Burning product will float on the surface and spread fire. Specific Hazards Arising from the Chemical: Extremely flammable aerosol. Contents under pressure. Keep away ...
... Suitable Extinguishing Media: Use water fog, dry chemical, carbon dioxide or foam. Do not use water jet or flooding amounts of water. Burning product will float on the surface and spread fire. Specific Hazards Arising from the Chemical: Extremely flammable aerosol. Contents under pressure. Keep away ...
Lecture 7
... The metals are strong reducing agents and react with most nonmetals producing ionic products The reaction of the metals with water is also a redox reaction. ...
... The metals are strong reducing agents and react with most nonmetals producing ionic products The reaction of the metals with water is also a redox reaction. ...
Comparison of 2008 to 2000 SCH3U_ud
... local populations [IP, PR, AI, C] Sample issue: Base metal smelting produces useful metals such as zinc, lead, copper, and nickel directly from their ores. However, during smelting, harmful compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all ...
... local populations [IP, PR, AI, C] Sample issue: Base metal smelting produces useful metals such as zinc, lead, copper, and nickel directly from their ores. However, during smelting, harmful compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all ...
Chemistry Fall Final Study Guide Concepts
... 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the ...
... 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the ...
chem A exercise package C
... regions, such as for oxygen, will result in the gain of two electrons. This process of overlapping atoms is called covalent bonding. The substance that results from covalent bonding is called a covalent substance. The process of overlapping atoms will keep occurring for a particular atom until it ha ...
... regions, such as for oxygen, will result in the gain of two electrons. This process of overlapping atoms is called covalent bonding. The substance that results from covalent bonding is called a covalent substance. The process of overlapping atoms will keep occurring for a particular atom until it ha ...
SCH 3U - mquagliaoths
... 43) Predicting whether or not a single displacement reaction will occur requires one to know which element is the more reactive – the one on its own or the one in a compound. Predicting whether a double displacement reaction involving the formation of a precipitate will occur involves knowing whethe ...
... 43) Predicting whether or not a single displacement reaction will occur requires one to know which element is the more reactive – the one on its own or the one in a compound. Predicting whether a double displacement reaction involving the formation of a precipitate will occur involves knowing whethe ...
Prospects for monitoring freshwater ecosystems towards the
... Multiple indicators for measuring freshwater condition have been tailored to different systems around the world, and in fact the science and practice of freshwater indicator development is relatively advanced compared with work in the terrestrial and marine realms. However, developing and applying s ...
... Multiple indicators for measuring freshwater condition have been tailored to different systems around the world, and in fact the science and practice of freshwater indicator development is relatively advanced compared with work in the terrestrial and marine realms. However, developing and applying s ...
Final Review 2
... d) None of the above is correct. 61) Which of the following is not one of Dalton’s laws? a) Atoms are indestructible. b) Atoms of the same element have isotopes with different masses. c) Atoms of different elements have different chemical and physical properties. d) All of these are examples of Dalt ...
... d) None of the above is correct. 61) Which of the following is not one of Dalton’s laws? a) Atoms are indestructible. b) Atoms of the same element have isotopes with different masses. c) Atoms of different elements have different chemical and physical properties. d) All of these are examples of Dalt ...
Chemical Formula Detective
... dispose of it in the waste container. Add 5 drops of 6M HCl to dissolve any insoluble aluminum salts and clear up the solution. 7. In the next steps, you will be collecting the copper by filtration. Secure a filtration flask (Erlenmeyer flask with a side port) to a ring stand using a large clamp. At ...
... dispose of it in the waste container. Add 5 drops of 6M HCl to dissolve any insoluble aluminum salts and clear up the solution. 7. In the next steps, you will be collecting the copper by filtration. Secure a filtration flask (Erlenmeyer flask with a side port) to a ring stand using a large clamp. At ...
2011-2012 Paper 1
... (b) Suggest a chemical test to distinguish each of the following pairs of solutions. Each test should include the reagent(s), the expected observation with each compound and the chemical equation(s) (i) ...
... (b) Suggest a chemical test to distinguish each of the following pairs of solutions. Each test should include the reagent(s), the expected observation with each compound and the chemical equation(s) (i) ...