- Kendriya Vidyalaya Jhunjhunu
... Three Marks Question 1. The oxide of a metal M was water soluble. When a blue litmus strip was dipped in this solution, it did not undergo any change in colour. Predict the nature of the oxide. 2. Why does bee-sting cause pain and irritation? What relief can be given in such a case immediately? 3. ...
... Three Marks Question 1. The oxide of a metal M was water soluble. When a blue litmus strip was dipped in this solution, it did not undergo any change in colour. Predict the nature of the oxide. 2. Why does bee-sting cause pain and irritation? What relief can be given in such a case immediately? 3. ...
1970 - Warren County Schools
... very little water from the ice. The solution now (d) Because of the polar nature of water, it is capable of solvating the ions that result from the dissociahas a freezing point lower than the temperature of tion, whereas the nonpolar benzene interacts very the ice, therefore, the ice melts. weakly w ...
... very little water from the ice. The solution now (d) Because of the polar nature of water, it is capable of solvating the ions that result from the dissociahas a freezing point lower than the temperature of tion, whereas the nonpolar benzene interacts very the ice, therefore, the ice melts. weakly w ...
Chemical Reactions
... A decomposition reaction is a reaction has one reactant, and two or more products. CaCO3(s) CaO(s) + CO2(g) ...
... A decomposition reaction is a reaction has one reactant, and two or more products. CaCO3(s) CaO(s) + CO2(g) ...
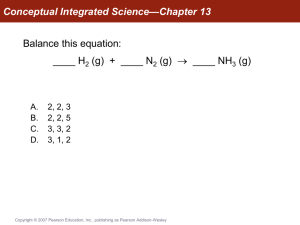
Conceptual Integrated Science—Chapter 13
... The warm air from a lit birthday candle does not rise within an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the ca ...
... The warm air from a lit birthday candle does not rise within an orbiting space station because there is no up or down. As a result, what happens to the burning candle and why? A. The warm air surrounding the candle speeds up the rate of reaction so that the candle burns brighter. B. Soot from the ca ...
chemistry
... 1.3.3 Natural/Man-made Many substances freely used these days are not available from natural sources, but this distinction is not at all useful for chemists, because it tells us little or nothing about the properties of the substance. Many natural substances can be man-made and samples from each sou ...
... 1.3.3 Natural/Man-made Many substances freely used these days are not available from natural sources, but this distinction is not at all useful for chemists, because it tells us little or nothing about the properties of the substance. Many natural substances can be man-made and samples from each sou ...
Chemical reaction
... of the substances. These are called stoichiometric coefficients and represent the number ratio of element and/or compound across a balanced chemical equation. ...
... of the substances. These are called stoichiometric coefficients and represent the number ratio of element and/or compound across a balanced chemical equation. ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... very little water from the ice. The solution now (d) Because of the polar nature of water, it is capable of solvating the ions that result from the dissociahas a freezing point lower than the temperature of tion, whereas the nonpolar benzene interacts very the ice, therefore, the ice melts. weakly w ...
... very little water from the ice. The solution now (d) Because of the polar nature of water, it is capable of solvating the ions that result from the dissociahas a freezing point lower than the temperature of tion, whereas the nonpolar benzene interacts very the ice, therefore, the ice melts. weakly w ...
Formatting Blackline Masters
... (sodium and chlorine). The properties of the salt differ from the properties of the elements. Salt is a compound. 2. Water (H2O) is a homogeneous material that can be decomposed into elements (hydrogen and oxygen). Water is a compound. 3. Copper is a homogeneous material that cannot be separated int ...
... (sodium and chlorine). The properties of the salt differ from the properties of the elements. Salt is a compound. 2. Water (H2O) is a homogeneous material that can be decomposed into elements (hydrogen and oxygen). Water is a compound. 3. Copper is a homogeneous material that cannot be separated int ...
Radiation Chemistry of Overirradiated Aqueous Solutions of
... molecules produced radiolytically; and (2) the radiation resistance of larger radiolytic products, in particular those releasing carboxylic and amino acids. It is known that hydrogen cyanide in irradiated solutions acts not only as the precursor of some compounds, but also as their protector. By sca ...
... molecules produced radiolytically; and (2) the radiation resistance of larger radiolytic products, in particular those releasing carboxylic and amino acids. It is known that hydrogen cyanide in irradiated solutions acts not only as the precursor of some compounds, but also as their protector. By sca ...
Organic and Bio-Molecular Chemistry
... systematic study which put into evidence the common characteristics of these compounds, that have been classified as “organic compounds”. For a long time it was believed that organic compounds were generated in Nature by a sort of magic “vital force”, despite the fact that in 1828 Wölher was able to ...
... systematic study which put into evidence the common characteristics of these compounds, that have been classified as “organic compounds”. For a long time it was believed that organic compounds were generated in Nature by a sort of magic “vital force”, despite the fact that in 1828 Wölher was able to ...
CHE 1402 Lab Manual
... 7. Do not put flammable liquids near an open flame. 8. When heating a test tube, make certain that the open end of the tube is directed away from the students. 9. When finished with your Bunsen Burner for a given portion of an experiment, turn it off. 10. Do not sit on the lab benches. 11. Do not en ...
... 7. Do not put flammable liquids near an open flame. 8. When heating a test tube, make certain that the open end of the tube is directed away from the students. 9. When finished with your Bunsen Burner for a given portion of an experiment, turn it off. 10. Do not sit on the lab benches. 11. Do not en ...
Chemistry Content Review Notes
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
Analytical Chemistry - University of Delhi
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
Chemical Dynamics at Surfaces
... The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea. ...
... The Haber process now produces 100 million tons of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea. ...
Unit 8 Student Notes
... ends of nearby water molecules; anions are attracted to the positive ends of nearby water molecules. If the attraction is strong enough, the anion will be pulled away from the surface of the crystal. This process is called dissolution (the general term for all solvents and solutes is dissociation). ...
... ends of nearby water molecules; anions are attracted to the positive ends of nearby water molecules. If the attraction is strong enough, the anion will be pulled away from the surface of the crystal. This process is called dissolution (the general term for all solvents and solutes is dissociation). ...
Extended Abstract
... chemical reactions of acids had been noted by Stephen Hales in his studies of plants, animals, and minerals. Lavoisier noted that effervescence produced a cooling effect and was not caused by frictional processes. He also studied melting ice and in 1771 noted that the temperature of an ice-water mix ...
... chemical reactions of acids had been noted by Stephen Hales in his studies of plants, animals, and minerals. Lavoisier noted that effervescence produced a cooling effect and was not caused by frictional processes. He also studied melting ice and in 1771 noted that the temperature of an ice-water mix ...
Untitled
... trademarks, nor does the use of such trademarks imply any affiliation with or endorsement of this book by such owners. ...
... trademarks, nor does the use of such trademarks imply any affiliation with or endorsement of this book by such owners. ...
Electrolyte Concentration Effect of a Photoelectrochemical Cell
... environmentally friend method is a very important issue. Hydrogen is used in fuel cells, but nature gas is still a main source for hydrogen production. It contains contaminative byproducts. Only a few part of hydrogen is produced by water splitting in the world [1]. Pollutant degradation is another ...
... environmentally friend method is a very important issue. Hydrogen is used in fuel cells, but nature gas is still a main source for hydrogen production. It contains contaminative byproducts. Only a few part of hydrogen is produced by water splitting in the world [1]. Pollutant degradation is another ...
industry: applying chemical reactions
... injuries and deaths are difficult to find. However, a few large fires have been reported, both in battery production plants and battery recycling facilities. Also, do you remember large recalls of lithium-ion computer batteries in the last several years? Laptops were bursting into flames due to the ...
... injuries and deaths are difficult to find. However, a few large fires have been reported, both in battery production plants and battery recycling facilities. Also, do you remember large recalls of lithium-ion computer batteries in the last several years? Laptops were bursting into flames due to the ...
Acid-Base Equilibria and Activity
... to which they react varies, and is quantified by an equilibrium constant. These constants for the weak acid and weak base hydrolysis reactions are labeled Ka and Kb , respectively. We’ll see how to use these constants to determine equilibrium concentrations later in this chapter. First we must be ab ...
... to which they react varies, and is quantified by an equilibrium constant. These constants for the weak acid and weak base hydrolysis reactions are labeled Ka and Kb , respectively. We’ll see how to use these constants to determine equilibrium concentrations later in this chapter. First we must be ab ...
CHEMISTRY
... An introduction to mathematical and statistical methods and techniques for analysis of data and results generated by various instrumental methods of chemical analysis (e.g., UV-visible, fluorescence, NMR, and FTIR spectrome tric methods, gas and liquid chromatographic methods, GC/MS, voltammetric me ...
... An introduction to mathematical and statistical methods and techniques for analysis of data and results generated by various instrumental methods of chemical analysis (e.g., UV-visible, fluorescence, NMR, and FTIR spectrome tric methods, gas and liquid chromatographic methods, GC/MS, voltammetric me ...
Photo-oxidation of pinonaldehyde at low NOx
... chemistry of pinonaldehyde and similar compounds in the literature (Glasius et al., 1997). Though we recently described SOA chemistry under highNOx conditions (Chacon-Madrid and Donahue, 2011), it is important to explore its chemistry in low-NOx conditions because products of biogenic species are co ...
... chemistry of pinonaldehyde and similar compounds in the literature (Glasius et al., 1997). Though we recently described SOA chemistry under highNOx conditions (Chacon-Madrid and Donahue, 2011), it is important to explore its chemistry in low-NOx conditions because products of biogenic species are co ...
Molecules, Moles and Chemical Equations File
... that releases the same amount of energy. Careful examination of the progress of explosive chemical reactions reveals that they accelerate as they proceed. As a result, all of the available explosive is consumed in a very short period of time. As that happens, the energy from the explosion is also re ...
... that releases the same amount of energy. Careful examination of the progress of explosive chemical reactions reveals that they accelerate as they proceed. As a result, all of the available explosive is consumed in a very short period of time. As that happens, the energy from the explosion is also re ...