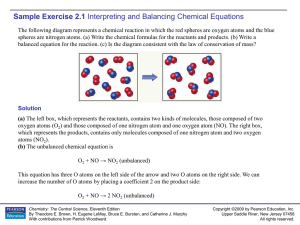
Sample Exercise 2.1
... (a) The symbol for lithium is Li. With the exception of mercury, all metals are solids at room temperature. Fluorine occurs as a diatomic molecule (see Figure 2.19). Thus, the reactants are Li(s) and F2(g). The product will be composed of a metal and a nonmetal, so we expect it to be an ionic solid. ...
... (a) The symbol for lithium is Li. With the exception of mercury, all metals are solids at room temperature. Fluorine occurs as a diatomic molecule (see Figure 2.19). Thus, the reactants are Li(s) and F2(g). The product will be composed of a metal and a nonmetal, so we expect it to be an ionic solid. ...
Supplemental information
... After 15 days of gravitational setting, 92% of the bare HFO particles had sedimented, compared with 1.9% and 2.5% sedimentation at CMC concentrations of 0.064 and 0.161 wt %, respectively. The results indicated that HFO nanoparticle suspensions prepared at final concentrations of 100 mg·L-1 as Fe an ...
... After 15 days of gravitational setting, 92% of the bare HFO particles had sedimented, compared with 1.9% and 2.5% sedimentation at CMC concentrations of 0.064 and 0.161 wt %, respectively. The results indicated that HFO nanoparticle suspensions prepared at final concentrations of 100 mg·L-1 as Fe an ...
selected experiments in organic chemistry
... 1. Throw all solids to be discarded into a waste paper basket. Never throw matches, filter paper, broken glass or any insoluble chemicals into the sink. Organic liquids should not be poured into the sink either. They are collected in special residue bottles. 2. Read the label twice before using a re ...
... 1. Throw all solids to be discarded into a waste paper basket. Never throw matches, filter paper, broken glass or any insoluble chemicals into the sink. Organic liquids should not be poured into the sink either. They are collected in special residue bottles. 2. Read the label twice before using a re ...
word - My eCoach
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
... When certain ionic solids crystallize from aqueous solutions, a definite number of molecules of water remain attached to the crystal. Ionic solids that contain a definite amount of water are called hydrates or hydrated salts and the water in the crystal structure is called water of hydration. The wa ...
Water Chemistry - U
... on our answers to the second question, and they are related to the broad goals we set for coverage of topics in this book. Although previous introductory water chemistry textbooks provide excellent coverage on inorganic equilibrium chemistry, they do not provide much coverage on other topics that ha ...
... on our answers to the second question, and they are related to the broad goals we set for coverage of topics in this book. Although previous introductory water chemistry textbooks provide excellent coverage on inorganic equilibrium chemistry, they do not provide much coverage on other topics that ha ...
Mole and Energy - Deans Community High School
... Avogadro’s constant, L or NA, is the number of elementary entities (particles) in one mole of any substance Avogadro’s constant = 6.02 x 1023 formula units ...
... Avogadro’s constant, L or NA, is the number of elementary entities (particles) in one mole of any substance Avogadro’s constant = 6.02 x 1023 formula units ...
Chemistry - Northeastern Illinois University
... Experience in chemistry in an off-campus location, e.g. business or government. The student registering selects well-defined academic goals to be achieved. These goals will be selected in cooperation with an on-campus advisor. Independent studies require the approval of the instructor, department ch ...
... Experience in chemistry in an off-campus location, e.g. business or government. The student registering selects well-defined academic goals to be achieved. These goals will be selected in cooperation with an on-campus advisor. Independent studies require the approval of the instructor, department ch ...
Stoichiometry File
... of chemistry involve the development of new routes to synthesize existing natural compounds. As you might imagine, making any of these syntheses commercially feasible requires detailed and quantitative understanding of the reactions involved. The economics of any chemical process obviously depend on ...
... of chemistry involve the development of new routes to synthesize existing natural compounds. As you might imagine, making any of these syntheses commercially feasible requires detailed and quantitative understanding of the reactions involved. The economics of any chemical process obviously depend on ...
A Simplified GuidE to Writing Watershed Restoration Plans In North
... In any watershed assessment (either simple or complex), stressors (causes), and sources need to be identified. The watershed assessment generally has greater value if the stressors and sources can be identified with as specific location(s) as possible (i.e., subwatershed, stream, stream segment). Th ...
... In any watershed assessment (either simple or complex), stressors (causes), and sources need to be identified. The watershed assessment generally has greater value if the stressors and sources can be identified with as specific location(s) as possible (i.e., subwatershed, stream, stream segment). Th ...
Writing Equilibrium Cons... and Liquids - Chemwiki
... There are all sorts of calculations you might be expected to do which are centered around equilibrium constants. You might be expected to calculate a value for including its units (which vary from case to case). Alternatively you might have to calculate equilibrium concentrations from a given valu ...
... There are all sorts of calculations you might be expected to do which are centered around equilibrium constants. You might be expected to calculate a value for including its units (which vary from case to case). Alternatively you might have to calculate equilibrium concentrations from a given valu ...
CHEMICAL EQUILIBRIUM
... 8. Even though the individual sets of equilibrium concentrations are quite different for the different situations, the equilibrium constant which depends on the ratio of the concentrations, remains the same. 9. Each set of equilibrium concentrations is called an ______________________________. 10. ...
... 8. Even though the individual sets of equilibrium concentrations are quite different for the different situations, the equilibrium constant which depends on the ratio of the concentrations, remains the same. 9. Each set of equilibrium concentrations is called an ______________________________. 10. ...
LaBrake, Fundamentals Diagnostic Questions
... These are questions to be used to help you fully prepare for 1A. While these topics will be covered in the 1ABC series, they will only be covered extremely briefly. It is expected that your chemistry background has prepared you to handle questions of this nature. Various sources can be used to help ...
... These are questions to be used to help you fully prepare for 1A. While these topics will be covered in the 1ABC series, they will only be covered extremely briefly. It is expected that your chemistry background has prepared you to handle questions of this nature. Various sources can be used to help ...
Acidic Environment by Ahmad Shah Idil
... 1. Indicators were identified with the observation that the colour of some flowers depends on soil composition: ...
... 1. Indicators were identified with the observation that the colour of some flowers depends on soil composition: ...
LABORATORY MANUAL FOR GENERAL CHEMISTRY I
... After you’ve read through the experiment, try to answer the review questions we’ve included at the end of each experiment. These questions will help you to understand the experiment in advance. Some of your experiments will also contain an element of danger. For this and other reasons, your lab inst ...
... After you’ve read through the experiment, try to answer the review questions we’ve included at the end of each experiment. These questions will help you to understand the experiment in advance. Some of your experiments will also contain an element of danger. For this and other reasons, your lab inst ...
Proton Transfers at the Air
... impossible; but I will give it a shot. I would like to start by thanking my thesis co-advisers, Professor Mike Hoffmann and Professor Bill Goddard, for providing me with boundless intellectual freedom, constant access, personalized mentoring, and continuous funding during my stay at Caltech. The ver ...
... impossible; but I will give it a shot. I would like to start by thanking my thesis co-advisers, Professor Mike Hoffmann and Professor Bill Goddard, for providing me with boundless intellectual freedom, constant access, personalized mentoring, and continuous funding during my stay at Caltech. The ver ...
Calculation of hydrogen isotopic fractionations in biogeochemical
... ranges for ␣1 and ␣2 should be overlapping but not identical. The form of Eqn. 13 leads to two additional considerations regarding experimental design. First, both parameters (p1, p2) can be calculated if the isotopic composition of only one hydrogen source is varied, and that of both hydrogen sourc ...
... ranges for ␣1 and ␣2 should be overlapping but not identical. The form of Eqn. 13 leads to two additional considerations regarding experimental design. First, both parameters (p1, p2) can be calculated if the isotopic composition of only one hydrogen source is varied, and that of both hydrogen sourc ...
9/10/10 1 Chemistry 121: Atomic and Molecular Chemistry
... The Mole and Molar Mass: Chemists measure atoms and molecules in moles. • In the SI system the mole (mol) is the amount of a substance that contains as many elementary entities (atoms, molecules, or other particles) as there are atoms in exactly 12 g (or 0.012 kg) of the carbon-12 isotope. The actu ...
... The Mole and Molar Mass: Chemists measure atoms and molecules in moles. • In the SI system the mole (mol) is the amount of a substance that contains as many elementary entities (atoms, molecules, or other particles) as there are atoms in exactly 12 g (or 0.012 kg) of the carbon-12 isotope. The actu ...
solliqsol - chemmybear.com
... (b) The rock salt forms a concentrated solution with very little water from the ice. The solution now has a freezing point lower than the temperature of the ice, therefore, the ice melts. (c) [question and answer in the GASES section] (d) Carbon dioxide is more dense than air and so pushes the air a ...
... (b) The rock salt forms a concentrated solution with very little water from the ice. The solution now has a freezing point lower than the temperature of the ice, therefore, the ice melts. (c) [question and answer in the GASES section] (d) Carbon dioxide is more dense than air and so pushes the air a ...
L A B O
... After you’ve read through the experiment, try to answer the review questions we’ve included at the end of each experiment. These questions will help you to understand the experiment in advance. Some of your experiments will also contain an element of danger. For this and other reasons, your lab inst ...
... After you’ve read through the experiment, try to answer the review questions we’ve included at the end of each experiment. These questions will help you to understand the experiment in advance. Some of your experiments will also contain an element of danger. For this and other reasons, your lab inst ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough there were two of them but my book had the mass of only one. Instead of a mass the other isotope had a β- by it, which is clearly wrong. The table I found is given below. Cou ...
... average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough there were two of them but my book had the mass of only one. Instead of a mass the other isotope had a β- by it, which is clearly wrong. The table I found is given below. Cou ...
FREE Sample Here - We can offer most test bank and
... 3. The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He concluded that the total weight did not change during a process. Which of these best describes Lavoisier's conclusion? a. From observation, Lavoisier cr ...
... 3. The French chemist Antoine Lavoisier found that the weight of objects before burning and the weight of the products after burning were equal. He concluded that the total weight did not change during a process. Which of these best describes Lavoisier's conclusion? a. From observation, Lavoisier cr ...
Recycling and Chemical Mathematics
... chickens, pigs, and goats, which supply eggs, meat, and milk, and as food for fish. The natural process that enables ...
... chickens, pigs, and goats, which supply eggs, meat, and milk, and as food for fish. The natural process that enables ...
CSEC Chemistry Revision Guide Answers.indd
... struck by a moving neutron, it splits into two smaller atoms. As it splits, two or three neutrons and a large amount of heat energy are released. The neutrons can then strike other atoms, causing them to split and release more neutrons and energy. This causes a chain reaction which releases very lar ...
... struck by a moving neutron, it splits into two smaller atoms. As it splits, two or three neutrons and a large amount of heat energy are released. The neutrons can then strike other atoms, causing them to split and release more neutrons and energy. This causes a chain reaction which releases very lar ...
LABORATORY MANUAL CHEMISTRY 121
... reaction can be followed visually because the products are red while the reactant is green. The purpose of this exercise is to determine the rate law for the hydrolysis of trans-[Co(en)2Cl2]+ and to determine the activation energy for the hydrolysis reaction by carrying out the reaction at several d ...
... reaction can be followed visually because the products are red while the reactant is green. The purpose of this exercise is to determine the rate law for the hydrolysis of trans-[Co(en)2Cl2]+ and to determine the activation energy for the hydrolysis reaction by carrying out the reaction at several d ...























