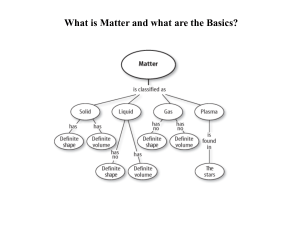
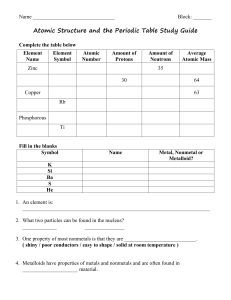
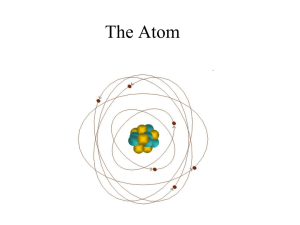
Posttest answers - Aurora City Schools
... Between the other two…on stair step line Properties in between…better conductors than nonmetals, not as good as metals… semiconductors. All are solid at room temp. Pretest additional questions There’s not a whole lot about ions and isotopes on the test, but you should be able to do this anyway. Also ...
... Between the other two…on stair step line Properties in between…better conductors than nonmetals, not as good as metals… semiconductors. All are solid at room temp. Pretest additional questions There’s not a whole lot about ions and isotopes on the test, but you should be able to do this anyway. Also ...
The Nuclear Atom
... weight of exactly 12.000000000 and is used as the basis upon which the atomic weight of other isotopes is determined ...
... weight of exactly 12.000000000 and is used as the basis upon which the atomic weight of other isotopes is determined ...
atomic theory quiz II review
... Review Sheet We will have a quiz over the information you have learned about the atomic theory. Please actively study (sing, dance, act, draw, write) the following info: ...
... Review Sheet We will have a quiz over the information you have learned about the atomic theory. Please actively study (sing, dance, act, draw, write) the following info: ...
Matter and the Periodic Table
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
Full Text PDF - Science and Education Publishing
... The answer to the aforementioned question is given by quantum electrodynamics (QED), a theory that combines quantum mechanics and special relativity, usually taught in Physics advanced courses. The basic idea is easy to grasp: electrons (especially the most internal ones) interacting with a heavy nu ...
... The answer to the aforementioned question is given by quantum electrodynamics (QED), a theory that combines quantum mechanics and special relativity, usually taught in Physics advanced courses. The basic idea is easy to grasp: electrons (especially the most internal ones) interacting with a heavy nu ...
Define:
... 98. How many electrons does strontium have to give up to achieve noble gas configuration? 99. What is the formula for the ion formed when sodium achieves noble gas electron configuration? ...
... 98. How many electrons does strontium have to give up to achieve noble gas configuration? 99. What is the formula for the ion formed when sodium achieves noble gas electron configuration? ...
Periodic Table Jeopardy
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
... Atomic Theory with evidence. He had four key postulates that he wanted everyone to know. ...
Unit2StudyGuide
... 17. What is a cation? Which particles does it have more of: electrons or protons? Average Atomic Mass 1. How do you calculate the average atomic mass of an element? 2. Example: The Carbon atom has three isotopes: Carbon-12 at 98.2%, Carbon-13 at 1.2%, and Carbon-14 at 0.6%. What is the average atomi ...
... 17. What is a cation? Which particles does it have more of: electrons or protons? Average Atomic Mass 1. How do you calculate the average atomic mass of an element? 2. Example: The Carbon atom has three isotopes: Carbon-12 at 98.2%, Carbon-13 at 1.2%, and Carbon-14 at 0.6%. What is the average atomi ...
Atomic Models 2015-2016
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
Extra Credit Test Review
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
study guide - atomic srtucture/_classification of matter
... Nuclear symbol: Show the element’s symbol, the atomic mass & the atomic number. Mass goes on the top left of the symbol, number goes on the bottom left of the symbol. mass#atomic number X Bohr Model: Shows number of electrons in each energy level around the nucleus. Mixtures vs. Substances ...
... Nuclear symbol: Show the element’s symbol, the atomic mass & the atomic number. Mass goes on the top left of the symbol, number goes on the bottom left of the symbol. mass#atomic number X Bohr Model: Shows number of electrons in each energy level around the nucleus. Mixtures vs. Substances ...
Define:
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
Atomic Theory
... were discovered, it was found that Mendeleev had closely predicted the properties of the ...
... were discovered, it was found that Mendeleev had closely predicted the properties of the ...
Chapter 3, Section 1 Inside an Atom
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
... number of protons. The atomic number is equal to the number of protons in that element. The atomic number is a unique identifying characteristic. ...
Periodic Table
... this concept because the valence electrons are the electrons involved in bonding. You determine the valence electrons by counting the "s" and "p" electrons in that period. You can determine that fluorine has seven valence electrons by going to the second period and count over seven times. How many b ...
... this concept because the valence electrons are the electrons involved in bonding. You determine the valence electrons by counting the "s" and "p" electrons in that period. You can determine that fluorine has seven valence electrons by going to the second period and count over seven times. How many b ...
The Periodic Table of Elements - PAMS-Doyle
... • They have been moved to the bottom to make the periodic table easier to read • First row is the lanthanide series, shiny, soft, malleable metals, that are conductive • The second row is the actinide series, all are radioactive and only the first four are present in nature • Elements numbered 92-11 ...
... • They have been moved to the bottom to make the periodic table easier to read • First row is the lanthanide series, shiny, soft, malleable metals, that are conductive • The second row is the actinide series, all are radioactive and only the first four are present in nature • Elements numbered 92-11 ...
Atomic Structure/Electrons
... 10. He discovered the electron and developed the “plum pudding” model of the atom. 11. His five postulates make up atomic theory. 12. His gold foil experiment led to his discovery of the nucleus. 13. He developed the planetary model of the atom, which described the light spectrum. 14. What is the sh ...
... 10. He discovered the electron and developed the “plum pudding” model of the atom. 11. His five postulates make up atomic theory. 12. His gold foil experiment led to his discovery of the nucleus. 13. He developed the planetary model of the atom, which described the light spectrum. 14. What is the sh ...
Notes
... in energy levels (shells). The first energy level may contain up to 2 electrons, the second energy level up to 8 electrons and the third up to 8 electrons. The electron arrangement of an atom can be shown clearly using a target picture e.g. 1st energy level 2nd energy level ...
... in energy levels (shells). The first energy level may contain up to 2 electrons, the second energy level up to 8 electrons and the third up to 8 electrons. The electron arrangement of an atom can be shown clearly using a target picture e.g. 1st energy level 2nd energy level ...
Linking Asteroids and Meteorites through Reflectance Spectroscopy
... • http://cosmiclog.msnbc.msn.com/_news/2011/01/ 26/5925968-see-the-worlds-smallest-periodictable ...
... • http://cosmiclog.msnbc.msn.com/_news/2011/01/ 26/5925968-see-the-worlds-smallest-periodictable ...
element - Mrs. Phillips` Physical Science Webpage
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
Earth Chemistry
... • Found by calculating the weighted average of the atomic masses of the naturally occurring isotopes ...
... • Found by calculating the weighted average of the atomic masses of the naturally occurring isotopes ...
Atomic Structure/Electrons
... 10. He discovered the electron and developed the “plum pudding” model. B 11. His five postulates make up atomic theory. A 12. His gold foil experiment led to his discovery of the nucleus. C 13. He developed the planetary model of the atom, which described the light spectrum. D 14. What is the shape ...
... 10. He discovered the electron and developed the “plum pudding” model. B 11. His five postulates make up atomic theory. A 12. His gold foil experiment led to his discovery of the nucleus. C 13. He developed the planetary model of the atom, which described the light spectrum. D 14. What is the shape ...