Lab B
... Homogeneous catalytic reactions are those in which both the substrate and the catalyst are in the same phase, and although there are common examples of such processes in organic chemistry (e.g. acid catalysis), this experiment introduces transition-metal-catalyzed reactions. Transition-metal based h ...
... Homogeneous catalytic reactions are those in which both the substrate and the catalyst are in the same phase, and although there are common examples of such processes in organic chemistry (e.g. acid catalysis), this experiment introduces transition-metal-catalyzed reactions. Transition-metal based h ...
Coordination Chemistry Prof. Debashis Ray Department of
... metal center. But, in actual sense these two nitrogens are also shows some bonding interactions with the magnesium because the pocket is there. And once it is fitted within the pocket, these two nitrogens are also showing some interactions. And, ultimately we can get the corresponding structure wher ...
... metal center. But, in actual sense these two nitrogens are also shows some bonding interactions with the magnesium because the pocket is there. And once it is fitted within the pocket, these two nitrogens are also showing some interactions. And, ultimately we can get the corresponding structure wher ...
Introduction to Kinetics and Equilibrium
... cis ֎ trans (at 400 °C) Kc is called the equilibrium constant. For Kc, species are expressed as M (Moles L‐1) Products in numerator, reactants in denominator. Products in numerator, reactants in denominator. Concentrations raised to their stoichiometric coefficients ...
... cis ֎ trans (at 400 °C) Kc is called the equilibrium constant. For Kc, species are expressed as M (Moles L‐1) Products in numerator, reactants in denominator. Products in numerator, reactants in denominator. Concentrations raised to their stoichiometric coefficients ...
Exam 1, Spring 2000
... (a) Which flask contains more molecules of gas? __________________ (b) In which flask is the pressure greater? __________________ (c) In which flask is the average kinetic energy of molecules greater or do they have the same average energy? __________________ (d) In which flask are the molecules mov ...
... (a) Which flask contains more molecules of gas? __________________ (b) In which flask is the pressure greater? __________________ (c) In which flask is the average kinetic energy of molecules greater or do they have the same average energy? __________________ (d) In which flask are the molecules mov ...
Chemical Equilibrium - Chemistry with Mrs. Caruso Let the Bonding
... Kc- Measures of __concentration_ are used in the calculation of this equilibrium constant. Kp- _partial pressures__are used in the calculation of this equilibrium. Relationship between Kp and Kc: Based on the ideal gas law: Therefore, Kp = Kc(RT)∆n Ex. At 327°C, the equilibrium concentrations are [C ...
... Kc- Measures of __concentration_ are used in the calculation of this equilibrium constant. Kp- _partial pressures__are used in the calculation of this equilibrium. Relationship between Kp and Kc: Based on the ideal gas law: Therefore, Kp = Kc(RT)∆n Ex. At 327°C, the equilibrium concentrations are [C ...
The highly preorganized ligands 1,10-Phenanthroline
... attachment to gadolinium, leaving one available coordination site for a water molecule This is of importance because the ability to shift the MRI signal is a function of the number of water molecules directly attached to gadolinium.8 An ideal contrast agent would not have as many points of attachmen ...
... attachment to gadolinium, leaving one available coordination site for a water molecule This is of importance because the ability to shift the MRI signal is a function of the number of water molecules directly attached to gadolinium.8 An ideal contrast agent would not have as many points of attachmen ...
Solubility Equilibria
... It is another example of applying Le Chatelier’s principle in solubility reactions. o Dissolution of ionic compounds containing OH ions are directly affected by the pH of the solution they are dissolved in. Increasing the pH by adding OH ...
... It is another example of applying Le Chatelier’s principle in solubility reactions. o Dissolution of ionic compounds containing OH ions are directly affected by the pH of the solution they are dissolved in. Increasing the pH by adding OH ...
M.Sc. Chemistry - Manonmaniam Sundaranar University
... CFT and LFT: Basic features of CFT and LFT. Splitting of the metal p– and d– orbitals in different CF symmetries like Td, Oh and square planar – Jahn - Teller distortion in Oh and Td complexes – static and dynamic J.T distortions. Application of CFT: Magnetic Properties, spectral properties, Spectro ...
... CFT and LFT: Basic features of CFT and LFT. Splitting of the metal p– and d– orbitals in different CF symmetries like Td, Oh and square planar – Jahn - Teller distortion in Oh and Td complexes – static and dynamic J.T distortions. Application of CFT: Magnetic Properties, spectral properties, Spectro ...
Metal Complexation of Thiacrown Ether Macrocycles by
... methanol/chloroform solutions as well as extractions of metals from aqueous solution by macrocycles in chloroform, it is found that the type of heteroatom (S, O, N), cavity size, and presence of other substituents influence the metal selectivities. Several of the macrocycles in this study bind mercu ...
... methanol/chloroform solutions as well as extractions of metals from aqueous solution by macrocycles in chloroform, it is found that the type of heteroatom (S, O, N), cavity size, and presence of other substituents influence the metal selectivities. Several of the macrocycles in this study bind mercu ...
Charge control of the inverse trans-influence
... complexes (2-t-Bu, 180.01 and 2-Ad, 175.8(3)1). The slight deviation from linearity in 2-Ad is due to the increased steric clash with the ortho-adamantyl ligand substituents. The uranium center lies 0.195 and 0.189 Å below the plane of the three aryloxide oxygen atoms for 2-t-Bu, while the out-of-pl ...
... complexes (2-t-Bu, 180.01 and 2-Ad, 175.8(3)1). The slight deviation from linearity in 2-Ad is due to the increased steric clash with the ortho-adamantyl ligand substituents. The uranium center lies 0.195 and 0.189 Å below the plane of the three aryloxide oxygen atoms for 2-t-Bu, while the out-of-pl ...
19-20 - TAMU Chemistry
... Water is a ligand itself so it can become involved in the chemistry: Overall reaction: [L5MX] + Y → [L5MY] + X 1.(slow) [L5MX] + H2O → [L5M(H2O)] + X 2. (fast) [L5M(H2O)] + Y → [L5MY] + H2O Two-steps, but one is very fast, so only the first one would contribute to the rate In the end, you may not ha ...
... Water is a ligand itself so it can become involved in the chemistry: Overall reaction: [L5MX] + Y → [L5MY] + X 1.(slow) [L5MX] + H2O → [L5M(H2O)] + X 2. (fast) [L5M(H2O)] + Y → [L5MY] + H2O Two-steps, but one is very fast, so only the first one would contribute to the rate In the end, you may not ha ...
273 - Wayne State Chemistry
... states relative to the ground states, and rotation along the N-GaPh3 vector without gallium-nitrogen bond cleavage. The activation parameters and exchange rates at 25 °C are similar, suggesting that the size of the pyrazolato carbon substituents has only a minor effect on the exchange process. To un ...
... states relative to the ground states, and rotation along the N-GaPh3 vector without gallium-nitrogen bond cleavage. The activation parameters and exchange rates at 25 °C are similar, suggesting that the size of the pyrazolato carbon substituents has only a minor effect on the exchange process. To un ...
Selected applications to organic synthesis of intramolecular C
... is much more puzzling and no rational explanation for its formation has yet been found. Another efficient way to activate the organopalladium compounds towards easy demetallation is to perform the reaction with cationic compounds (ref. 4d, f-i) so as to increase the electrophilicity of the palladium ...
... is much more puzzling and no rational explanation for its formation has yet been found. Another efficient way to activate the organopalladium compounds towards easy demetallation is to perform the reaction with cationic compounds (ref. 4d, f-i) so as to increase the electrophilicity of the palladium ...
P/S ligands derived from carbohydrates in Rh-catalyzed
... yield and 40% ee. Surprisingly, structurally related Rh(I) complex 5, with an adamantyl group instead of the tert-butyl moiety, leads to the secondary carbinol with a good 60% yield and 45% ee. In contrast, the conformationally and electronically different Rh(I)complex 6, still exhibits some limitat ...
... yield and 40% ee. Surprisingly, structurally related Rh(I) complex 5, with an adamantyl group instead of the tert-butyl moiety, leads to the secondary carbinol with a good 60% yield and 45% ee. In contrast, the conformationally and electronically different Rh(I)complex 6, still exhibits some limitat ...
Slajd 1
... A reversible process is one which can go back and forth between states along the same path. When 1 mol of water is frozen at 1 atm at 0 °C to form 1 mol of ice, q = ∆Hvap of heat is removed. To reverse the process, q = ∆Hvap must be added to the 1 mol of ice at 0°C and 1 atm to form 1 mol of water ...
... A reversible process is one which can go back and forth between states along the same path. When 1 mol of water is frozen at 1 atm at 0 °C to form 1 mol of ice, q = ∆Hvap of heat is removed. To reverse the process, q = ∆Hvap must be added to the 1 mol of ice at 0°C and 1 atm to form 1 mol of water ...
transition metals notes
... orbitals relative to the penetrating 4s orbitals. In the limit, the removal of enough screening electrons must return the relative orbital energies to the H atom sequence, with 3d << 4s. The vital point is that in all TM ions, all the valence electrons are d electrons. We can ignore the s shell. Eve ...
... orbitals relative to the penetrating 4s orbitals. In the limit, the removal of enough screening electrons must return the relative orbital energies to the H atom sequence, with 3d << 4s. The vital point is that in all TM ions, all the valence electrons are d electrons. We can ignore the s shell. Eve ...
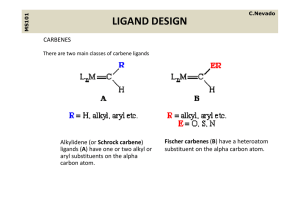
ligand design - UZH - Department of Chemistry
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...