Chapter 12 EDTA Titrations Coordination Number Geometries Ligands
... the analyte can be treated with excess Mg(EDTA)2-. The analyte displaces Mg, and than Mg can be titrated with standard EDTA • Indirect titration: Anions can be analyzed by precipitation with excess metal ion and then titration of the metal in the dissolved precipitate with EDTA. • Masking agent: pro ...
... the analyte can be treated with excess Mg(EDTA)2-. The analyte displaces Mg, and than Mg can be titrated with standard EDTA • Indirect titration: Anions can be analyzed by precipitation with excess metal ion and then titration of the metal in the dissolved precipitate with EDTA. • Masking agent: pro ...
ligand design - UZH - Department of Chemistry
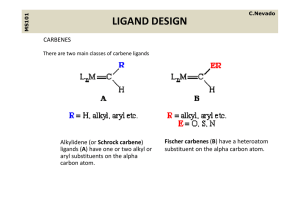
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...
Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of
... window coatings [11]. Many types of catalysis utilizing tin species have been reported [12], including hydrogenations [13], cross-coupling reactions [14], oxidation of carbon monoxide [15] and ethanol [16] and the reduction of carbon dioxide [17] and NOx [18]. Tin oxides [19] and tin sulfides [20] a ...
... window coatings [11]. Many types of catalysis utilizing tin species have been reported [12], including hydrogenations [13], cross-coupling reactions [14], oxidation of carbon monoxide [15] and ethanol [16] and the reduction of carbon dioxide [17] and NOx [18]. Tin oxides [19] and tin sulfides [20] a ...
1984 Advanced Placement Exam
... 50. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 gram of hydrogen and the other holds 8.0 grams of oxygen. Which of the following statements regarding these gas samples is FALSE? (A) The volume of the hydrogen container is the same as the volume of the o ...
... 50. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 gram of hydrogen and the other holds 8.0 grams of oxygen. Which of the following statements regarding these gas samples is FALSE? (A) The volume of the hydrogen container is the same as the volume of the o ...
Low-temperature lifetimes of metastable high
... becomes less dramatic, following the bell shaped curve of Marcus theory with, in principle, a maximum value as n approaches S [15]. 2.2. The low-temperature tunnelling rate constant and extrapolation to low-spin complexes As mentioned above, for spin-crossover compounds the thermal transition temper ...
... becomes less dramatic, following the bell shaped curve of Marcus theory with, in principle, a maximum value as n approaches S [15]. 2.2. The low-temperature tunnelling rate constant and extrapolation to low-spin complexes As mentioned above, for spin-crossover compounds the thermal transition temper ...
Text - Reading`s CentAUR
... the organic phase by forming charge-neutral complexes with TBP (i.e., [UO2(TBP)2(NO3)2] and [Pu(TBP)2(NO3)4]).1,3−5 The plutonium, after reduction to Pu3+, and uranium are then back-extracted into an aqueous phase for reuse. The aqueous phase remaining after the initial separation, known as highly a ...
... the organic phase by forming charge-neutral complexes with TBP (i.e., [UO2(TBP)2(NO3)2] and [Pu(TBP)2(NO3)4]).1,3−5 The plutonium, after reduction to Pu3+, and uranium are then back-extracted into an aqueous phase for reuse. The aqueous phase remaining after the initial separation, known as highly a ...
Calculating molar volume
... This number is known as the Avogadro Constant. The Avogadro Constant can be found in the SQA data booklet and is expressed as 6.02 x 1023 mol-1. The term formula unit is a general term that relates to the type of particles that make up a substance. In general, it refers to the formula normally used ...
... This number is known as the Avogadro Constant. The Avogadro Constant can be found in the SQA data booklet and is expressed as 6.02 x 1023 mol-1. The term formula unit is a general term that relates to the type of particles that make up a substance. In general, it refers to the formula normally used ...
Trends in Physical Properties
... Chlorine behaves as an oxidising agent in the extraction of bromine from seawater. In this process, chlorine gas is bubbled through a solution containing bromide ions. (i) ...
... Chlorine behaves as an oxidising agent in the extraction of bromine from seawater. In this process, chlorine gas is bubbled through a solution containing bromide ions. (i) ...
Lecture 18. Chemical Equilibrium (Ch. 5)
... required. Haber Process, developed into an industrial process by C. Bosch - a major chemical breakthrough at the beginning of the 20th century (1909): T = 5000C, P = 250 bar, plus a catalist (!!!). At this temperature, K = 6.9·10-5 (the drop of K can be calculated using van’t Hoff’s equation and ΔH0 ...
... required. Haber Process, developed into an industrial process by C. Bosch - a major chemical breakthrough at the beginning of the 20th century (1909): T = 5000C, P = 250 bar, plus a catalist (!!!). At this temperature, K = 6.9·10-5 (the drop of K can be calculated using van’t Hoff’s equation and ΔH0 ...
Co-ordination engineering: when can one speak of an
... other three are presented here. Generally, a bridging coordination mode gives rise to a one-dimensional metal–ligand substructure. Bridge formation together with metal chelation results in a two-dimensional metal–ligand substructure. Depending on the anion, both types of substructures can then be co ...
... other three are presented here. Generally, a bridging coordination mode gives rise to a one-dimensional metal–ligand substructure. Bridge formation together with metal chelation results in a two-dimensional metal–ligand substructure. Depending on the anion, both types of substructures can then be co ...
C2 Additional Chemistry Thursday 14 May
... an alkaline solution. It is used to produce ammonium salts, which are important as fertilisers. Recall that the pH scale is a measure of the acidity or alkalinity of a solution. Describe an acid as releasing H+ ions in solution. Describe an alkali as releasing OH- ions in solution. In neutralisation ...
... an alkaline solution. It is used to produce ammonium salts, which are important as fertilisers. Recall that the pH scale is a measure of the acidity or alkalinity of a solution. Describe an acid as releasing H+ ions in solution. Describe an alkali as releasing OH- ions in solution. In neutralisation ...
Metal–dioxygen and metal–dinitrogen complexes: where are the
... Side-on bound dioxygen often bridges to an additional metal through a second side-on interaction, and the resulting binding mode is described as m-h2 :h2 or “side-on/side-on” binding (Fig. 1). The most intense characterization of this binding mode for O2 complexes has come in m-h2 :h2 -peroxodicoppe ...
... Side-on bound dioxygen often bridges to an additional metal through a second side-on interaction, and the resulting binding mode is described as m-h2 :h2 or “side-on/side-on” binding (Fig. 1). The most intense characterization of this binding mode for O2 complexes has come in m-h2 :h2 -peroxodicoppe ...
Chapter 17 - Cengage Learning
... Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction t ...
... Increases in the temperature and concentration of reactants bring about more collisions, and the rate of reaction increases. The collision model explains many observations about reactions. Not all molecules that collide react. Colliding molecules must have a minimum amount of energy for a reaction t ...
Oxygen Dissociation by Concerted Action of Di
... oxygen, respectively. In these STM topographs, some of the diiron units in the pristine phase described above are still present: The metal centers are highlighted by purple circles and their TPA ligands by magenta cross lines. The interaction with O2 induces drastic modifications, and after 27 L very ...
... oxygen, respectively. In these STM topographs, some of the diiron units in the pristine phase described above are still present: The metal centers are highlighted by purple circles and their TPA ligands by magenta cross lines. The interaction with O2 induces drastic modifications, and after 27 L very ...
MS PowerPoint - Catalysis Eprints database
... bonds, and can only undergo sigma to sigma* transitions) shows an absorbance maximum at 125 nm. Absorption maxima due to sigma to sigma* transitions are not seen in typical UV-Vis. spectra (200 - 700 nm) n to sigma* Transitions Saturated compounds containing atoms with lone pairs (non-bonding electr ...
... bonds, and can only undergo sigma to sigma* transitions) shows an absorbance maximum at 125 nm. Absorption maxima due to sigma to sigma* transitions are not seen in typical UV-Vis. spectra (200 - 700 nm) n to sigma* Transitions Saturated compounds containing atoms with lone pairs (non-bonding electr ...
Solution Notes, Molarity, Dilutions
... – Polar & Ionic • Water and most Salts (NaCl, CaCl2, KI, etc.) ...
... – Polar & Ionic • Water and most Salts (NaCl, CaCl2, KI, etc.) ...
nanyang technological university entrance examination syllabus for
... Periodicity of chemical properties of the elements in the third period (i) Reaction of the elements with oxygen and chlorine (ii) Variation in oxidation number of the oxides (sodium to sulfur only) and of the chlorides (sodium to phosphorus only) (iii) Reactions of these oxides and chlorides with wa ...
... Periodicity of chemical properties of the elements in the third period (i) Reaction of the elements with oxygen and chlorine (ii) Variation in oxidation number of the oxides (sodium to sulfur only) and of the chlorides (sodium to phosphorus only) (iii) Reactions of these oxides and chlorides with wa ...