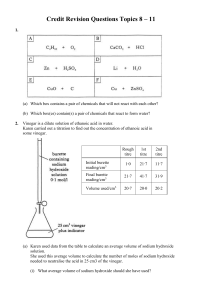
Credit Revision Questions Topics 8 – 11 1. (a) Which box contains a
... (b) (i) What term is used to describe the type of chemical reaction taking place in beaker B? (ii) Suggest what would happen to the pH in beaker B. (c) Write the ion-electron equation for the chemical reaction taking place in beaker A. 10. Some Euro coins are made from a hard-wearing alloy called No ...
... (b) (i) What term is used to describe the type of chemical reaction taking place in beaker B? (ii) Suggest what would happen to the pH in beaker B. (c) Write the ion-electron equation for the chemical reaction taking place in beaker A. 10. Some Euro coins are made from a hard-wearing alloy called No ...
msc_pre_chemistry_pap1_bl3
... The study of the complexes is supposed to be incomplete without finding the stability or formation constants, because most of the properties and utility of the complexes depend on it. The value of stability constants may predict the conditions required for complete formation of a given complex. This ...
... The study of the complexes is supposed to be incomplete without finding the stability or formation constants, because most of the properties and utility of the complexes depend on it. The value of stability constants may predict the conditions required for complete formation of a given complex. This ...
Chap 24. Transition Metals and Coordination Compounds
... Bonding in a complex ion is considered to be an electrostatic attraction between the positively charged nucleus of the central metal ion and electrons in the ligands. Repulsions also occur between the ligand electrons and electrons in the central ion. Crystal field theory focuses on the repulsions b ...
... Bonding in a complex ion is considered to be an electrostatic attraction between the positively charged nucleus of the central metal ion and electrons in the ligands. Repulsions also occur between the ligand electrons and electrons in the central ion. Crystal field theory focuses on the repulsions b ...
Document
... Stable complexes have a large POSITIVE GoRXN for ligand substitution and Inert complexes have a large POSITIVE G‡ (activation). ...
... Stable complexes have a large POSITIVE GoRXN for ligand substitution and Inert complexes have a large POSITIVE G‡ (activation). ...
Synthesis and Characteristic Study of Co(II), Ni(II
... oxygen of the carbonyl group of the five-member ring and the Schiff base behaves as a bidentate ligand. 3-from the molar conductivity data suggest that uncharged the coordination sphere, and presence of chloride ion binding with Co(II), Ni(II) and Cu(II) ions inside the coordination sphere. 4-The mo ...
... oxygen of the carbonyl group of the five-member ring and the Schiff base behaves as a bidentate ligand. 3-from the molar conductivity data suggest that uncharged the coordination sphere, and presence of chloride ion binding with Co(II), Ni(II) and Cu(II) ions inside the coordination sphere. 4-The mo ...
Chapter 20: Reactions of Complexes (Mechanisms)
... concentration of A, [A]0, and plotting log d[A]/dt vs. log[A]0, gives the reaction order a of reactant A as the slope and log k’ as the y‐intercept Only the rate determining step may be directly measured by traditional means ...
... concentration of A, [A]0, and plotting log d[A]/dt vs. log[A]0, gives the reaction order a of reactant A as the slope and log k’ as the y‐intercept Only the rate determining step may be directly measured by traditional means ...
Metal Ions
... proteins allowing ions to transfer between the inside and outside of the cell 4. Electrical signals are propagated down the axon by the difference in charge between the outside of the cell and the inside of the cell 5. When a neuron is at rest, a. Inside of cell is negative b. There are lots of pota ...
... proteins allowing ions to transfer between the inside and outside of the cell 4. Electrical signals are propagated down the axon by the difference in charge between the outside of the cell and the inside of the cell 5. When a neuron is at rest, a. Inside of cell is negative b. There are lots of pota ...
American-Journal-of-Oil-and-Chemical-Technologies
... In conclusion, continuing with our previous works on synthesizing supramolecular compounds containing pyridinedicarboxylic acid N-oxides [9-11], two new coordination complexes have been synthesized and characterized. The red shift of bands ʋas(COO−), ʋs(COO−), and ʋas(COO−) and ʋ(NO) confirm formati ...
... In conclusion, continuing with our previous works on synthesizing supramolecular compounds containing pyridinedicarboxylic acid N-oxides [9-11], two new coordination complexes have been synthesized and characterized. The red shift of bands ʋas(COO−), ʋs(COO−), and ʋas(COO−) and ʋ(NO) confirm formati ...
Transition Elements
... A molecule of oxaliplatin has a square planar shape about the metal ion with two bidentate ligands. The structure of oxaliplatin is shown below. H2 N ...
... A molecule of oxaliplatin has a square planar shape about the metal ion with two bidentate ligands. The structure of oxaliplatin is shown below. H2 N ...
Crystal Field Theory
... spectrum reveals that this transition occurs with a maximum at 20300 cm-1 which corresponds to ∆o 243 kJ/mol. (1000 cm-1 = 11.96 kJ/mol or 2.86 kcal/mol or 0.124 eV.) ...
... spectrum reveals that this transition occurs with a maximum at 20300 cm-1 which corresponds to ∆o 243 kJ/mol. (1000 cm-1 = 11.96 kJ/mol or 2.86 kcal/mol or 0.124 eV.) ...
Applications of CFT for Oh Complexes 1. High- and low
... • hard to see but dx2-y2 and dz2 point between the ligands more than do dxz, dyz and dxy so they are relatively stabilized • the tetrahedral crystal field (Δt) splitting is less than octahedral splitting (Δoct) because no orbitals point directly at the ligands: ...
... • hard to see but dx2-y2 and dz2 point between the ligands more than do dxz, dyz and dxy so they are relatively stabilized • the tetrahedral crystal field (Δt) splitting is less than octahedral splitting (Δoct) because no orbitals point directly at the ligands: ...
File
... • As many as 6 N (as NH3) could bond directly to Co3+ • Cl- could bond to Co3+ or associate loosely; two kinds of Cl- ...
... • As many as 6 N (as NH3) could bond directly to Co3+ • Cl- could bond to Co3+ or associate loosely; two kinds of Cl- ...
Inorganic Chemistry: Fundamental Principals as Applied to the
... (Fig. 5). The difference in energy between the two sets of orbitals (Δoct) is determined both by the metal and by the ligands coordinating the metal. This spacing, in turn, determines the order in which the d orbitals are filled with electrons. Ligands that induce large values of Δoct are called “st ...
... (Fig. 5). The difference in energy between the two sets of orbitals (Δoct) is determined both by the metal and by the ligands coordinating the metal. This spacing, in turn, determines the order in which the d orbitals are filled with electrons. Ligands that induce large values of Δoct are called “st ...
Stability of Coordination Compounds
... Equilibrium constants defined as shown above are termed formation constants or stability constants. Those for the reverse reaction are instability or dissociation constants. There can be some confusion here because in certain areas of chemistry and biology the term “stability constant” actually is ...
... Equilibrium constants defined as shown above are termed formation constants or stability constants. Those for the reverse reaction are instability or dissociation constants. There can be some confusion here because in certain areas of chemistry and biology the term “stability constant” actually is ...
B.Sc III weekly planner 2016
... Crystal field theory inTetrahedral complexes, calculation of CFSE. Crystal field theory in tetragonal and square planar complexes Factors determining the magnitude of crystal field splitting, colours of transition metal complexes Limitations of crystal field theory, comparision of valence bond theor ...
... Crystal field theory inTetrahedral complexes, calculation of CFSE. Crystal field theory in tetragonal and square planar complexes Factors determining the magnitude of crystal field splitting, colours of transition metal complexes Limitations of crystal field theory, comparision of valence bond theor ...























