Chapter 1 Structure and Bonding
... iii. CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh. iv. PtA2B2 only has two isomers. It must be square planar rather than tetrahedral, which would only have 1 isomer. v. ...
... iii. CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh. iv. PtA2B2 only has two isomers. It must be square planar rather than tetrahedral, which would only have 1 isomer. v. ...
Transition Elements/Coordination Chemistry
... Valence Bond theory: Bonding takes place when the filled atomic orbital on the ligand overlaps an empty atomic orbital on the metal ion. Explains geometries well, but doesn’t explain color or magnetic properties. Crystal Field theory: Bonds form due to the attraction of the electrons on the ligand f ...
... Valence Bond theory: Bonding takes place when the filled atomic orbital on the ligand overlaps an empty atomic orbital on the metal ion. Explains geometries well, but doesn’t explain color or magnetic properties. Crystal Field theory: Bonds form due to the attraction of the electrons on the ligand f ...
Instructions for Preparing Manuscript for Bulletin of
... give greater importance than other methods dosage hepatitis to assess the state of deficiency, or toxicity sufficient. Stored mainly in parenchymal cells, copper may occur in greater quantity and Kupffer cells in case of poisoning. Biological role of Copper amino acid In biological systems, copper i ...
... give greater importance than other methods dosage hepatitis to assess the state of deficiency, or toxicity sufficient. Stored mainly in parenchymal cells, copper may occur in greater quantity and Kupffer cells in case of poisoning. Biological role of Copper amino acid In biological systems, copper i ...
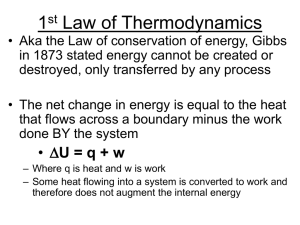
Lecture 5 - Thermodynamics II
... • Entropy is a measure of the disorder (randomness) of a system. The higher the entropy of the system, the more disordered it is. • The second law states that the universe always becomes more disordered in any real process. • The entropy (order) of a system can decrease, but in order for this to hap ...
... • Entropy is a measure of the disorder (randomness) of a system. The higher the entropy of the system, the more disordered it is. • The second law states that the universe always becomes more disordered in any real process. • The entropy (order) of a system can decrease, but in order for this to hap ...
File
... • Acid – H+ ion (or H3O+) is only positive ion in soln –H+ ion is also called a PROTON –H3O+ is called a HYDRONIUM ion ...
... • Acid – H+ ion (or H3O+) is only positive ion in soln –H+ ion is also called a PROTON –H3O+ is called a HYDRONIUM ion ...
Chapter 1 Structure and Bonding
... 2) s-donors only a) en > NH3 because it is more basic (stronger field ligand) b) F- > Cl- > Br- > I- (basicity) 3) p-donors a) Halides field strength is lowered due to p-donor ability b) For similar reasons H2O, OH-, RCO2- also are weak field ligands 4) p-acceptors increase ligand field strength: CO ...
... 2) s-donors only a) en > NH3 because it is more basic (stronger field ligand) b) F- > Cl- > Br- > I- (basicity) 3) p-donors a) Halides field strength is lowered due to p-donor ability b) For similar reasons H2O, OH-, RCO2- also are weak field ligands 4) p-acceptors increase ligand field strength: CO ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... the ligand and its metal complexes made us to prepare some of the new metal complexes. ...
... the ligand and its metal complexes made us to prepare some of the new metal complexes. ...
Early transition metal clusters with π
... books on the chemistry of metal clusters do not break with this tradition. Following a book concentrating on the first category [(1990). The Chemistry of Metal Cluster Complexes, edited by D. F Shriver, H. D. Kaesz and R. D. Adams. Weinheim: VCH], this companion volume focuses on the second. The tre ...
... books on the chemistry of metal clusters do not break with this tradition. Following a book concentrating on the first category [(1990). The Chemistry of Metal Cluster Complexes, edited by D. F Shriver, H. D. Kaesz and R. D. Adams. Weinheim: VCH], this companion volume focuses on the second. The tre ...
Transition Metal Chemistry
... Elemental iron reacts with non-oxidizing acids to form Fe2+, which oxidizes in air to Fe3+ ...
... Elemental iron reacts with non-oxidizing acids to form Fe2+, which oxidizes in air to Fe3+ ...
pH Scale and Concentration Date: Chemistry!
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
Metal Complexes of N-hydroxyethylnaphthalideneimine Schiff Base
... [{CI0H6(O)CH = =N(CH2)2OH}2Mn] [{CI0H6(O)CH= =N(OH2)2OH}2CO] [{C10H6(O)CH = =N(CH2)2OH}2Ni] [{CI0H6(O)CH = =N(CH2)20H}2CU] [{C10H6(O)CH = =N(CH2)2OH}2Zn] [{CI0H6(O)CH = =N(CH2)2OH }2Cd] [{CI0H6(O)CH = =N(CH2)20H}2Pd] [{CI0H6(O)CH = =N(CH2)2OH}2UOo] ...
... [{CI0H6(O)CH = =N(CH2)2OH}2Mn] [{CI0H6(O)CH= =N(OH2)2OH}2CO] [{C10H6(O)CH = =N(CH2)2OH}2Ni] [{CI0H6(O)CH = =N(CH2)20H}2CU] [{C10H6(O)CH = =N(CH2)2OH}2Zn] [{CI0H6(O)CH = =N(CH2)2OH }2Cd] [{CI0H6(O)CH = =N(CH2)20H}2Pd] [{CI0H6(O)CH = =N(CH2)2OH}2UOo] ...
1.1 Werner`s Coordination Theory 1.2 Coordination
... If the metal complex is an anion, the suffix –ate is added to the metal name a. If necessary, drop -ium, -en, -ese ending before adding –ate b. “cobaltate”, “nickelate”, “chromate” (not chromiumate), “manganate” (not manganeseate) c. What if the metal is iron, gold, or copper? Following the metal’s ...
... If the metal complex is an anion, the suffix –ate is added to the metal name a. If necessary, drop -ium, -en, -ese ending before adding –ate b. “cobaltate”, “nickelate”, “chromate” (not chromiumate), “manganate” (not manganeseate) c. What if the metal is iron, gold, or copper? Following the metal’s ...
Slide 1
... Dihydrogen, CH bonds in alkyls groups and alkanes and some other electron-rich -bonds (Si-H, B-H, etc.) can. A simple way to illustrate the ability of H2 to serve as a 2e-donor is given below. H3+ is a know species that forms from H2 and H+: H3+ 2 H + H+ ...
... Dihydrogen, CH bonds in alkyls groups and alkanes and some other electron-rich -bonds (Si-H, B-H, etc.) can. A simple way to illustrate the ability of H2 to serve as a 2e-donor is given below. H3+ is a know species that forms from H2 and H+: H3+ 2 H + H+ ...
Final Exam S06
... The complex [Ni(CN)4]2- is diamagnetic, but [NiCl4]2- is paramagnetic with two unpaired electrons. Use the magnetic behavior of these complexes to deduce the geometric structures, i.e. molecular geometry, of each of these species. In recording your answer, be sure to also provide the name of each mo ...
... The complex [Ni(CN)4]2- is diamagnetic, but [NiCl4]2- is paramagnetic with two unpaired electrons. Use the magnetic behavior of these complexes to deduce the geometric structures, i.e. molecular geometry, of each of these species. In recording your answer, be sure to also provide the name of each mo ...
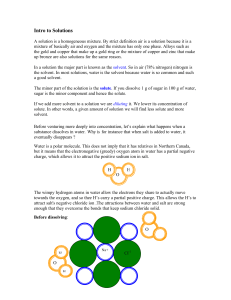
Solutions Intro
... towards the oxygen, and so thee H’s carry a partial positive charge. This allows the H’s to attract salt's negative chloride ion .The attractions between water and salt are strong enough that they overcome the bonds that keep sodium chloride solid. ...
... towards the oxygen, and so thee H’s carry a partial positive charge. This allows the H’s to attract salt's negative chloride ion .The attractions between water and salt are strong enough that they overcome the bonds that keep sodium chloride solid. ...
Complexing properties of bivalent and trivalent iron in the system
... Perchlorate was determined chromatometrically by differential Potentiometrie titration [15]. Iron(III) Perchlorate was made by dissolving freshly prepared iron(III) hydroxide in dilute anal, grade perchloric acid. The concentration of iron(III) Perchlorate was determined with complexone III by diffe ...
... Perchlorate was determined chromatometrically by differential Potentiometrie titration [15]. Iron(III) Perchlorate was made by dissolving freshly prepared iron(III) hydroxide in dilute anal, grade perchloric acid. The concentration of iron(III) Perchlorate was determined with complexone III by diffe ...