Synthesis, spectral characterization of schiff base alkaline earth
... The analytical data for the ligand and complexes together with some physical properties are summarized in table 1. The data from complexes correspond well with the general formula ML, where M = Ba, Ca & Sr; L = C46H58 N4O4. The magnetic susceptibilities of the complexes at room temperature were cons ...
... The analytical data for the ligand and complexes together with some physical properties are summarized in table 1. The data from complexes correspond well with the general formula ML, where M = Ba, Ca & Sr; L = C46H58 N4O4. The magnetic susceptibilities of the complexes at room temperature were cons ...
I. Introduction. In this section we consider "simple" electrochemistry
... Calculated values of .1G:~ Ao and AI for some .Inorgamc ...
... Calculated values of .1G:~ Ao and AI for some .Inorgamc ...
H - Chemical Biology Research Group
... • In the solution phase electrostatic + dispersion and inductive forces (London and Debye) also important • In nature (e.g. Lys-Trp, Arg-Phe etc.) ...
... • In the solution phase electrostatic + dispersion and inductive forces (London and Debye) also important • In nature (e.g. Lys-Trp, Arg-Phe etc.) ...
National Interest in Minerals
... Denotes most common oxidation states in solution at neutral pH ...
... Denotes most common oxidation states in solution at neutral pH ...
tutorials 1-5
... 7. Show that the formula for an infinite layered lattice of edge-shared octahedra is AB3 where A is at the center of each octahedron. 8. Na0.99Cl0.99 will have Schottky or Frenkel defects. Explain. 9. At high temperatures the defect concentration increases. Is there a thermodynamic basis for the abo ...
... 7. Show that the formula for an infinite layered lattice of edge-shared octahedra is AB3 where A is at the center of each octahedron. 8. Na0.99Cl0.99 will have Schottky or Frenkel defects. Explain. 9. At high temperatures the defect concentration increases. Is there a thermodynamic basis for the abo ...
ELECTRON COUNTING IN TRANSITION METAL COMPLEXES
... charge on the structure. We don't have to do that here, because we already make the adjustment when we decide the "oxidation state" or charge on the metal. ...
... charge on the structure. We don't have to do that here, because we already make the adjustment when we decide the "oxidation state" or charge on the metal. ...
Section 14.1
... Equilibria Involving Complex Ions In the chemical equilibrium of nickel ions, ammonia, and water, the complex ions have different colors. You can tell which ion has the greater concentration based on color: [Ni(H2O)6]2+(aq) + 6 NH3(aq) ...
... Equilibria Involving Complex Ions In the chemical equilibrium of nickel ions, ammonia, and water, the complex ions have different colors. You can tell which ion has the greater concentration based on color: [Ni(H2O)6]2+(aq) + 6 NH3(aq) ...
1. Copper(I) Chloride
... In concentrated HCl solution, the dichlorocuprate ion, [CuCl2]–, is the actual product: Cu(s) + Cu2+(aq) + 4 Cl–(aq) ֖ 2 [CuCl2]–(aq) ...
... In concentrated HCl solution, the dichlorocuprate ion, [CuCl2]–, is the actual product: Cu(s) + Cu2+(aq) + 4 Cl–(aq) ֖ 2 [CuCl2]–(aq) ...
UV-vis-spectrometry applications, Flow Injection Analysis
... 5) One ml of 2,4-dinitrophenylhydrazine reagent is added immediately, thereby stopping the reaction. 6) After the tube is permitted to stand at room temperature for a minimum of 20 min, 7) 10 ml of 0.4N NaOH are added, a rubber stopper is inserted, and the contents are mixed by ...
... 5) One ml of 2,4-dinitrophenylhydrazine reagent is added immediately, thereby stopping the reaction. 6) After the tube is permitted to stand at room temperature for a minimum of 20 min, 7) 10 ml of 0.4N NaOH are added, a rubber stopper is inserted, and the contents are mixed by ...
Topic 18 - Coordination Compounds
... A. Coordination compound A coordination compound is a neutral species consisting of one or more complex ions. B. Complex ion 1. A complex ion is an ion with a central metal ion bonded to one or more molecules or ions. 2. The central atom is a Lewis acid. 3. The attached molecules or ions are ligands ...
... A. Coordination compound A coordination compound is a neutral species consisting of one or more complex ions. B. Complex ion 1. A complex ion is an ion with a central metal ion bonded to one or more molecules or ions. 2. The central atom is a Lewis acid. 3. The attached molecules or ions are ligands ...
Acids and Bases and Aqueous Equilibria
... Gilbert Newton Lewis (1875-1946) In a 1923 paper, Lewis wrote: "We are so habituated to the use of water as a solvent, and our data are so frequently limited to those obtained in aqueous solutions, that we frequently define an acid or a base as a substance whose aqueous solution gives, respectively, ...
... Gilbert Newton Lewis (1875-1946) In a 1923 paper, Lewis wrote: "We are so habituated to the use of water as a solvent, and our data are so frequently limited to those obtained in aqueous solutions, that we frequently define an acid or a base as a substance whose aqueous solution gives, respectively, ...
Homework 1 - IONiC / VIPEr
... valence shell (remember that metals lose their s electrons first; if there are any s-electrons on the metal, (Cu(I) for example) then these count for the purposes of dn count, since they are in the valence shell). The hardest to count is the total valence electrons, and 2 methods for doing this are ...
... valence shell (remember that metals lose their s electrons first; if there are any s-electrons on the metal, (Cu(I) for example) then these count for the purposes of dn count, since they are in the valence shell). The hardest to count is the total valence electrons, and 2 methods for doing this are ...
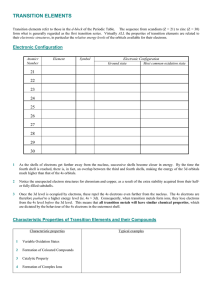
TRANSITION METALS - Pennsylvania State University
... Sc3+, Ti4+ are stable (maximum oxidation states). Sc2O3 Stable oxide. Mn7+ Exists but is easily reduced. MnO4Strong oxidizing agent. ...
... Sc3+, Ti4+ are stable (maximum oxidation states). Sc2O3 Stable oxide. Mn7+ Exists but is easily reduced. MnO4Strong oxidizing agent. ...
Periodic Table
... Paramagnetism --- Molecules with one or more unpaired electrons are attracted to a magnetic field. The more unpaired electrons in the molecule the stronger the attraction. This type of behavior is called Diamagnetism --- Substances with no unpaired electrons are weakly repelled by a magnetic field. ...
... Paramagnetism --- Molecules with one or more unpaired electrons are attracted to a magnetic field. The more unpaired electrons in the molecule the stronger the attraction. This type of behavior is called Diamagnetism --- Substances with no unpaired electrons are weakly repelled by a magnetic field. ...
Transition Metals - wellswaysciences
... ethanedioate ions form one dative bond from each N in the molecule so are bidentate. • These can form octahedral complexes with cis-trans isomers as long as 2 bidentate ligands are present with 2 monodentate ligands, e.g. [CoCl2(en)2] or [Cr(C2O4)2(H2O)2]• EDTA is a chelating hexadentate ligand – ch ...
... ethanedioate ions form one dative bond from each N in the molecule so are bidentate. • These can form octahedral complexes with cis-trans isomers as long as 2 bidentate ligands are present with 2 monodentate ligands, e.g. [CoCl2(en)2] or [Cr(C2O4)2(H2O)2]• EDTA is a chelating hexadentate ligand – ch ...
What Should Be Impossible: Resolution of the Mononuclear Gallium
... Chirality of metal complexes has been of theoretical interest to chemists since the time of Werner2 and the relative stability of such complexes was explained by Taube’s application of crystal field theory.3 Metal complex chirality also has important practical applications in areas as varied as chir ...
... Chirality of metal complexes has been of theoretical interest to chemists since the time of Werner2 and the relative stability of such complexes was explained by Taube’s application of crystal field theory.3 Metal complex chirality also has important practical applications in areas as varied as chir ...
Copper(II) Mixed Ligands Complexes of Hydroxamic Acids with
... free, sodium hydroxide solutions were used in all titrations. The electrode system (1) was calibrated for hydrogen ion concentration12, before and after each series of measurements, by titration of perchloric acid (or sodium hydroxide) with standardized sodium hydroxide solution (or perchloric acid ...
... free, sodium hydroxide solutions were used in all titrations. The electrode system (1) was calibrated for hydrogen ion concentration12, before and after each series of measurements, by titration of perchloric acid (or sodium hydroxide) with standardized sodium hydroxide solution (or perchloric acid ...