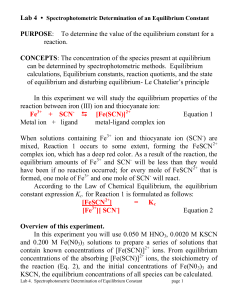
PURPOSE: To determine the value of the equilibrium constant for a
... and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between positively charged ions and negatively charged ions or negatively charged lone pair of electrons. Comple ...
... and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between positively charged ions and negatively charged ions or negatively charged lone pair of electrons. Comple ...
2000 us national chemistry olympiad
... This is a multiple-choice examination with four choices for each question. There is only one correct or best answer to each question. When you select your choice, blacken the corresponding space on the answer sheet with your pencil. Make a heavy full mark, but no stray marks. If you decide to change ...
... This is a multiple-choice examination with four choices for each question. There is only one correct or best answer to each question. When you select your choice, blacken the corresponding space on the answer sheet with your pencil. Make a heavy full mark, but no stray marks. If you decide to change ...
PDF
... the extinction coefficients for the left and right circular polarized light, εL and εD, respectively, may differ depending upon the wavelength (λ) of the polarized incident beam. Therefore the difference between the extinction coefficients, Δε = εL - εD, may not be zero and Δε is called circular dic ...
... the extinction coefficients for the left and right circular polarized light, εL and εD, respectively, may differ depending upon the wavelength (λ) of the polarized incident beam. Therefore the difference between the extinction coefficients, Δε = εL - εD, may not be zero and Δε is called circular dic ...
Document
... 8. (a) Define reaction of formation and reaction of combustion. (b) Write sown the combustion reaction for phenol. Ans: The standard enthalpy of formation of phenol (C6H5OH) is −165.0 kJ mol−1. The standard enthalpy of formation H2O(l) is −285.83 kJ mol−1 The standard enthalpy of formation CO2(g) is ...
... 8. (a) Define reaction of formation and reaction of combustion. (b) Write sown the combustion reaction for phenol. Ans: The standard enthalpy of formation of phenol (C6H5OH) is −165.0 kJ mol−1. The standard enthalpy of formation H2O(l) is −285.83 kJ mol−1 The standard enthalpy of formation CO2(g) is ...
Chapter 13 - "Water and Solutions"
... like that of the CCI4 molecule illustrated here. Note that the carbon atom is in the center of the pyramid, with chlorine atoms at each of the four corners. (C) A water molecule has two bonding pairs and two lone pairs in a tetrahedral arrangement. (D) The water molecule has an angular arrangement c ...
... like that of the CCI4 molecule illustrated here. Note that the carbon atom is in the center of the pyramid, with chlorine atoms at each of the four corners. (C) A water molecule has two bonding pairs and two lone pairs in a tetrahedral arrangement. (D) The water molecule has an angular arrangement c ...
2.4 Examples 2.4.1 Nuclei of Low Abundance: Satellite Spectra To
... (which is greater for P, S and Se donors). It should be noted that the effect of the paramagnetic shielding term is not only restricted to quadrupolar nuclei. Any species with low energy paramagnetic excited states can exhibit this effect. Thus 103Rh (I = ½ ) also has a very wide chemical shift rang ...
... (which is greater for P, S and Se donors). It should be noted that the effect of the paramagnetic shielding term is not only restricted to quadrupolar nuclei. Any species with low energy paramagnetic excited states can exhibit this effect. Thus 103Rh (I = ½ ) also has a very wide chemical shift rang ...
calculations of mechanical work in 1, 2 and 3 dimensions
... A chemical reaction with methanol creates heat in the NiTi alloy wire. At a higher temperature, the wire’s length shrinks - thus lifting a weight and doing work on the surroundings. V.H. Ebron et al., Science (2006) 311, 1580 ...
... A chemical reaction with methanol creates heat in the NiTi alloy wire. At a higher temperature, the wire’s length shrinks - thus lifting a weight and doing work on the surroundings. V.H. Ebron et al., Science (2006) 311, 1580 ...
CHAPTER-7 EQUILIBRIUM Equilibrium state- When
... The acid-base pair thatdiffers only by one proton is called a conjugateacidbase pair. IfBrönsted acid is a strong acid then itsconjugate base is a weak base and viceversa. Ionic product of water.Kw = [H+][OH–] pH = -log [H+] ; here[H+] is molar concentration of hydrogen ion. pH + pOH =14 p ...
... The acid-base pair thatdiffers only by one proton is called a conjugateacidbase pair. IfBrönsted acid is a strong acid then itsconjugate base is a weak base and viceversa. Ionic product of water.Kw = [H+][OH–] pH = -log [H+] ; here[H+] is molar concentration of hydrogen ion. pH + pOH =14 p ...
CHM1 Review for Exam 9 Topics 1. Reaction Types a. Combustion
... a. __ C2H6 (g) + __ O2 (g) __ CO2 (g) + __ H2O (g) b. __ C2H6OH (g) + __ O2 (g) __ CO2 (g) + __ H2O (g) c. __ Ca(NO3)2 (aq) + __ Na3PO4 (aq) __ Ca3(PO4)2 (s) + __ NaNO3 (aq) d. __ CuCl2 (aq) + __ AgNO3 (aq) __ AgCl (s) + __ Cu(NO3)2 (aq) ...
... a. __ C2H6 (g) + __ O2 (g) __ CO2 (g) + __ H2O (g) b. __ C2H6OH (g) + __ O2 (g) __ CO2 (g) + __ H2O (g) c. __ Ca(NO3)2 (aq) + __ Na3PO4 (aq) __ Ca3(PO4)2 (s) + __ NaNO3 (aq) d. __ CuCl2 (aq) + __ AgNO3 (aq) __ AgCl (s) + __ Cu(NO3)2 (aq) ...
Synthesis and characterization of a nano Cu2 cluster
... (20/5, v/v) and added drop-wise to the previous mixture. The mixture was stirred vigorously for half an hour with step-wise addition of NaN3 (2mmol). The heterogenous reaction mixture was filtered and the deep greenish filtrate was stored at ambient condition. In four weeks, green single crystals we ...
... (20/5, v/v) and added drop-wise to the previous mixture. The mixture was stirred vigorously for half an hour with step-wise addition of NaN3 (2mmol). The heterogenous reaction mixture was filtered and the deep greenish filtrate was stored at ambient condition. In four weeks, green single crystals we ...
mod 2 revision guide 6. group 2
... slightly alkaline (pH 9) so some hydroxide ions must therefore have been produced by a very slight dissolving. Magnesium hydroxide is used in medicine (in suspension as milk of magnesia) to neutralise excess acid in the stomach and to treat constipation. Mg(OH)2 + 2HCl ...
... slightly alkaline (pH 9) so some hydroxide ions must therefore have been produced by a very slight dissolving. Magnesium hydroxide is used in medicine (in suspension as milk of magnesia) to neutralise excess acid in the stomach and to treat constipation. Mg(OH)2 + 2HCl ...
Specific borane electron counting I - The School of Life Sciences at
... been investigated, but many of these studies have been called into question because of the lack of structural verification.3 It is, therefore, significant that a variety of transition-metal complexes that feature MfB dative bonds have been recently structurally characterized.4-6 In each case, the ke ...
... been investigated, but many of these studies have been called into question because of the lack of structural verification.3 It is, therefore, significant that a variety of transition-metal complexes that feature MfB dative bonds have been recently structurally characterized.4-6 In each case, the ke ...
15transmpp
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
Dissociation
... As we have seen previously, substances which can conduct an electrical current in solution (because they dissociate to yield mobile ions) are known as electrolytes Any compound which exists mostly as dissociated ions in solution, rather than just as dissolved (but undissociated) molecules, is referr ...
... As we have seen previously, substances which can conduct an electrical current in solution (because they dissociate to yield mobile ions) are known as electrolytes Any compound which exists mostly as dissociated ions in solution, rather than just as dissolved (but undissociated) molecules, is referr ...
Review of Basic Principles: The following was adapted from The
... compared to the sp 3 hybrid of the methyl group. The remaining shortening of 0.32 Å is still substantial. We now need to confirm that it really is the π* orbital of CO that is involved in the back bonding. To do this we turn to IR (infrared) spectroscopy. If CO were bound to the metal by its carbon ...
... compared to the sp 3 hybrid of the methyl group. The remaining shortening of 0.32 Å is still substantial. We now need to confirm that it really is the π* orbital of CO that is involved in the back bonding. To do this we turn to IR (infrared) spectroscopy. If CO were bound to the metal by its carbon ...
The s-Block Elements
... reacts slowly with water. This illustrates the importance of the role of kinetic factors in determining the rate of a chemical reaction. Lithium has a higher m.p., this increases the activation energy required for dissolution in aqueous solution. It does not melt during the reaction as Na and K do, ...
... reacts slowly with water. This illustrates the importance of the role of kinetic factors in determining the rate of a chemical reaction. Lithium has a higher m.p., this increases the activation energy required for dissolution in aqueous solution. It does not melt during the reaction as Na and K do, ...
Slide 1
... Suppose you have a piece of magnesium in a beaker of water. There will be some tendency for the magnesium atoms to shed electrons and go into solution as magnesium ions. The electrons will be left behind on the magnesium In a very short time, there will be a build-up of electrons on the magnesium, a ...
... Suppose you have a piece of magnesium in a beaker of water. There will be some tendency for the magnesium atoms to shed electrons and go into solution as magnesium ions. The electrons will be left behind on the magnesium In a very short time, there will be a build-up of electrons on the magnesium, a ...