Experiment 22
... add 1 M NaOH to 1 M HCl, we will initially raise [OH-] ions way above 1 10-14 M. However, Reaction 3 cannot be in equilibrium when both [H+] and [OH-] are high; reaction must occur to re-establish equilibrium. The added OH- ions react-with H+ ions to form H2O, decreasing both concentrations until ...
... add 1 M NaOH to 1 M HCl, we will initially raise [OH-] ions way above 1 10-14 M. However, Reaction 3 cannot be in equilibrium when both [H+] and [OH-] are high; reaction must occur to re-establish equilibrium. The added OH- ions react-with H+ ions to form H2O, decreasing both concentrations until ...
Worksheet answers
... acids ionize in water to form H+ ions more precisely, the H from the acid molecule is donated to a water molecule to form hydronium ion, H3O+. A proton (H+) cannot exist on its own in water! bases dissociate in water to form OH ions bases, such as NH3, that do not contain OH ions, produce OH by p ...
... acids ionize in water to form H+ ions more precisely, the H from the acid molecule is donated to a water molecule to form hydronium ion, H3O+. A proton (H+) cannot exist on its own in water! bases dissociate in water to form OH ions bases, such as NH3, that do not contain OH ions, produce OH by p ...
Cobalt(III) as a Stable and Inert Mediator Ion between NTA and
... for [CoIIINTA(imidazole)2] (lmax = 543 nm). The formation of the [CoIIINTA(His6-peptide)] complex was further confirmed by ESI-MS. Four other His6-tagged proteins were also successfully immobilized on the Co3+-NTA agarose beads using 20 mm H2O2, which shows the generality of this approach (Figure S3 ...
... for [CoIIINTA(imidazole)2] (lmax = 543 nm). The formation of the [CoIIINTA(His6-peptide)] complex was further confirmed by ESI-MS. Four other His6-tagged proteins were also successfully immobilized on the Co3+-NTA agarose beads using 20 mm H2O2, which shows the generality of this approach (Figure S3 ...
Thermodynamics and Kinetics of Solids 21 ________________________________________________________________________________________________________________________
... vapor at a rate which depends on the relative pressure and flow rate. The vapor is condensed at a lower temperature and the mass is determined. Other Heterogeneous Equilibria Systems which contain more than 1 gas. Consideration of reactions between one gas and one condensed phase with the formation ...
... vapor at a rate which depends on the relative pressure and flow rate. The vapor is condensed at a lower temperature and the mass is determined. Other Heterogeneous Equilibria Systems which contain more than 1 gas. Consideration of reactions between one gas and one condensed phase with the formation ...
A-level Paper 3 Practice Paper 3 - A
... A flue-gas desulfurisation process involves the oxidation, by oxygen, of aqueous sulfate(IV) ions (SO3 2–) into aqueous sulfate(VI) ions (SO4 2–). This reaction is catalysed by Co2+ ions in an acidic aqueous solution. Write an equation for the overall reaction of sulfate(IV) ions with oxygen to form ...
... A flue-gas desulfurisation process involves the oxidation, by oxygen, of aqueous sulfate(IV) ions (SO3 2–) into aqueous sulfate(VI) ions (SO4 2–). This reaction is catalysed by Co2+ ions in an acidic aqueous solution. Write an equation for the overall reaction of sulfate(IV) ions with oxygen to form ...
Paramagnetic Metal Complexes as Water Proton
... of the agent is usually a simple function of its tissue concentration. For NMR relaxation agents, however, this requirement should be qualified: it is sufficient only that the relaxation rates of the target tissue be enhanced in preference to other tissues. This might be accomplished by means other ...
... of the agent is usually a simple function of its tissue concentration. For NMR relaxation agents, however, this requirement should be qualified: it is sufficient only that the relaxation rates of the target tissue be enhanced in preference to other tissues. This might be accomplished by means other ...
chem 304 inorganic chemistry laboratory manual
... situation is complicated further in that optical activity is also possible in the above case.) The number of ions constituting the complex is best determined by measuring the conductivity of the solution of that compound. This conductivity measurement allows one to tell how many ions (cations and an ...
... situation is complicated further in that optical activity is also possible in the above case.) The number of ions constituting the complex is best determined by measuring the conductivity of the solution of that compound. This conductivity measurement allows one to tell how many ions (cations and an ...
- Catalyst
... Oxidized form can pick up electrons; reduced form can release electrons elsewhere as pH or other conditions change. Example: Iron-Sulfur Proteins. Ancient; found in all organisms from bacteria to mammals. Tetrahedral FeS4 active site. Catalyze metabolic redox reactions. Cycle between Fe+3 and Fe+2, ...
... Oxidized form can pick up electrons; reduced form can release electrons elsewhere as pH or other conditions change. Example: Iron-Sulfur Proteins. Ancient; found in all organisms from bacteria to mammals. Tetrahedral FeS4 active site. Catalyze metabolic redox reactions. Cycle between Fe+3 and Fe+2, ...
Word - chemmybear.com
... the conditions of the experiment. Br2(g) + Cl2(g) 2 BrCl(g) If 0.180 moles of bromine gas and 0.180 moles of chlorine gas are introduced into a 3.0 Liter flask and allowed to come to equilibrium, what is the equilibrium concentration of the bromine monochloride? How much BrCl is produced? 13. When a ...
... the conditions of the experiment. Br2(g) + Cl2(g) 2 BrCl(g) If 0.180 moles of bromine gas and 0.180 moles of chlorine gas are introduced into a 3.0 Liter flask and allowed to come to equilibrium, what is the equilibrium concentration of the bromine monochloride? How much BrCl is produced? 13. When a ...
South Pasadena · AP Chemistry
... the conditions of the experiment. Br2(g) + Cl2(g) 2 BrCl(g) If 0.180 moles of bromine gas and 0.180 moles of chlorine gas are introduced into a 3.0 Liter flask and allowed to come to equilibrium, what is the equilibrium concentration of the bromine monochloride? How much BrCl is produced? 13. When a ...
... the conditions of the experiment. Br2(g) + Cl2(g) 2 BrCl(g) If 0.180 moles of bromine gas and 0.180 moles of chlorine gas are introduced into a 3.0 Liter flask and allowed to come to equilibrium, what is the equilibrium concentration of the bromine monochloride? How much BrCl is produced? 13. When a ...
Oxidative degradation of Potassium Dioxalato Diaquo Chromate (III)
... Manganese dioxide has been most extensively studied due to its good oxidizing property [1-4] and reasonably high catalytic activity [5-9]. It is one of the most extensively used heterogeneous catalysts for a variety of organic and inorganic transformations in gaseous as well as liquid phases. Howeve ...
... Manganese dioxide has been most extensively studied due to its good oxidizing property [1-4] and reasonably high catalytic activity [5-9]. It is one of the most extensively used heterogeneous catalysts for a variety of organic and inorganic transformations in gaseous as well as liquid phases. Howeve ...
Get Day 18 - Mattson Creighton
... Day 18. Transition Metals Complexes IV: Ligand Field Theory continued ...
... Day 18. Transition Metals Complexes IV: Ligand Field Theory continued ...
Pi-Acid handout - U of L Class Index
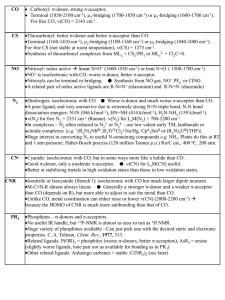
... Dinitrogen: isoelectronic with CO ● Worse s-donor and much worse p-acceptor than CO. A poor ligand, and very unreactive due to extremely strong N≡N triple bond. N-N bond dissociation energies: N≡N (946 kJmol-1), HN=NH (414 kJmol-1), H2N-NH2 (159 kJmol-1). n(N2) for free N2 = 2331 cm-1 (Raman). n( ...
... Dinitrogen: isoelectronic with CO ● Worse s-donor and much worse p-acceptor than CO. A poor ligand, and very unreactive due to extremely strong N≡N triple bond. N-N bond dissociation energies: N≡N (946 kJmol-1), HN=NH (414 kJmol-1), H2N-NH2 (159 kJmol-1). n(N2) for free N2 = 2331 cm-1 (Raman). n( ...
Document
... An unknown white solid is discovered on the lab counter in room 2101. Miss Allen wants to know if it is ionic or covalent. Describe how you could use its properties to determine if it is ionic or covalent. Be sure to use at least 3 specific examples of properties (3 marks) ...
... An unknown white solid is discovered on the lab counter in room 2101. Miss Allen wants to know if it is ionic or covalent. Describe how you could use its properties to determine if it is ionic or covalent. Be sure to use at least 3 specific examples of properties (3 marks) ...
Metal-induced tautomerization of oxazole and thiazole molecules to
... Oxazole (1a) and thiazole (1b) are simple molecules of great relevance in heterocyclic chemistry and important structural elements in many natural products.1 Their corresponding carbene tautomers, 2,3-dihydrooxazol-2-ylidene (2a) and 2,3-dihydrothiazol-2-ylidene (2b), derived from a 1,2-hydrogen shi ...
... Oxazole (1a) and thiazole (1b) are simple molecules of great relevance in heterocyclic chemistry and important structural elements in many natural products.1 Their corresponding carbene tautomers, 2,3-dihydrooxazol-2-ylidene (2a) and 2,3-dihydrothiazol-2-ylidene (2b), derived from a 1,2-hydrogen shi ...
Aspects of Coordination Chemistry (Part 2) Isomers in Coordination
... New labels are introduced to reflect the relative positions of the ligands around the octahedral structure. Thus; placing the 3 groups on one face of the ocathedral gives rise to the facial isomer and placing the 3 groups around the centre gives rise to the meridinal isomer. Draw the structures of ...
... New labels are introduced to reflect the relative positions of the ligands around the octahedral structure. Thus; placing the 3 groups on one face of the ocathedral gives rise to the facial isomer and placing the 3 groups around the centre gives rise to the meridinal isomer. Draw the structures of ...
The synthesis and characterization of a cationic technetium
... cationic complex H[Tc(NO)Cl4], the most commonly employed of the technetium-nitrosyl precursors. The other frequently employed synthon is the Tc(II) complex [TcCl3(NO)(PPh3)2], which is synthesized from (Bu4N)[Tc(NO)Cl4]. Since the established preparation of (Bu4N)[Tc(NO)Cl4] requires a full day to ...
... cationic complex H[Tc(NO)Cl4], the most commonly employed of the technetium-nitrosyl precursors. The other frequently employed synthon is the Tc(II) complex [TcCl3(NO)(PPh3)2], which is synthesized from (Bu4N)[Tc(NO)Cl4]. Since the established preparation of (Bu4N)[Tc(NO)Cl4] requires a full day to ...
Common Oxidizing Agents
... solutions of lithium hydroxide and hydrobromic acid are mixed. How does the size of the bromide ion compare to the chloride ion on the periodic table? ans: OH- + H+→ H2O. Br- is bigger than Cl-. ...
... solutions of lithium hydroxide and hydrobromic acid are mixed. How does the size of the bromide ion compare to the chloride ion on the periodic table? ans: OH- + H+→ H2O. Br- is bigger than Cl-. ...
NOMENCLATURE OF IONIC COMPOUNDS CHEMISTRY 1411
... (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in group 2 have a fixed oxidation number of +2. ...
... (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in group 2 have a fixed oxidation number of +2. ...
chapters 16-17 test re
... 3. _______ A low Ea means that relatively few collisions will have the required energy to produce the activated complex, and the reaction rate is fast. 4. _______ Catalysts are enzymes that aren’t consumed in a chemical reaction, but they raise the reaction rate by lowering the Ea. 5. _______ To cal ...
... 3. _______ A low Ea means that relatively few collisions will have the required energy to produce the activated complex, and the reaction rate is fast. 4. _______ Catalysts are enzymes that aren’t consumed in a chemical reaction, but they raise the reaction rate by lowering the Ea. 5. _______ To cal ...
Steps for writing Lewis structures
... e) In most acids, such as H2SO4, and in many other compounds that contain both oxygen and hydrogen atoms, the hydrogen atoms are all bonded to oxygen atoms. 2. Find the total number of valence electrons. Add together the number of valence electrons contributed by each atom. If the species is an ion, ...
... e) In most acids, such as H2SO4, and in many other compounds that contain both oxygen and hydrogen atoms, the hydrogen atoms are all bonded to oxygen atoms. 2. Find the total number of valence electrons. Add together the number of valence electrons contributed by each atom. If the species is an ion, ...