Review Package KCI 2017 Sem 1
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...
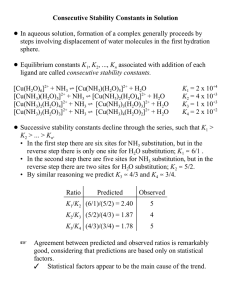
Consecutive Stability Constants in Solution
... Synthetic Use of the Trans Effect ! The synthetic utility of the trans effect can be illustrated by the selective production of cis or trans Pt(NH3)2Cl2, using the greater trans-directing ability of Cl– relative to NH3. To make cis-Pt(NH3)2Cl2, start with [PtCl4]2–: ...
... Synthetic Use of the Trans Effect ! The synthetic utility of the trans effect can be illustrated by the selective production of cis or trans Pt(NH3)2Cl2, using the greater trans-directing ability of Cl– relative to NH3. To make cis-Pt(NH3)2Cl2, start with [PtCl4]2–: ...
Worksheet
... (E) The positive charge of an atom is concentrated in a small region. 10 HI + 2 KMnO4 + 3 H2SO4 5 I2 + 2 MnSO4 + K2SO4 + 8 H2O 38. According to the balanced equation above, how many moles of HI would be necessary to produce 2.5 mol of I2, starting with 4.0 mol of KMnO4 and 3.0 mol of H2SO4? (A) 20 ...
... (E) The positive charge of an atom is concentrated in a small region. 10 HI + 2 KMnO4 + 3 H2SO4 5 I2 + 2 MnSO4 + K2SO4 + 8 H2O 38. According to the balanced equation above, how many moles of HI would be necessary to produce 2.5 mol of I2, starting with 4.0 mol of KMnO4 and 3.0 mol of H2SO4? (A) 20 ...
Contents and Concepts Learning Objectives
... a. Define ion product. b. State the criterion for precipitation. c. Predict whether precipitation will occur, given ion concentrations. d. Predict whether precipitation will occur, given solution volumes and concentrations. e. Define fractional precipitation. f. Explain how two ions can be separated ...
... a. Define ion product. b. State the criterion for precipitation. c. Predict whether precipitation will occur, given ion concentrations. d. Predict whether precipitation will occur, given solution volumes and concentrations. e. Define fractional precipitation. f. Explain how two ions can be separated ...
Chapter 1: Fundamental Concepts
... Stoichiometry: 1 mole Cr3+ ~ 3 moles e– ~ 1 mole Cr – If a current equivalent to 3 moles of electrons is passed through the solution of Cr3+, then 1 mole of Cr metal will be ...
... Stoichiometry: 1 mole Cr3+ ~ 3 moles e– ~ 1 mole Cr – If a current equivalent to 3 moles of electrons is passed through the solution of Cr3+, then 1 mole of Cr metal will be ...
Slide 1
... The M/M bond order is a function of transition metal electron configuration. The Table below is valid for the case when metal dx2-y2 orbital is used for s-bonding with ligands. ...
... The M/M bond order is a function of transition metal electron configuration. The Table below is valid for the case when metal dx2-y2 orbital is used for s-bonding with ligands. ...
Samples - About OSN Academy
... proton with a charge +1 and one orbital electron. The electronic structure may be written as 1s1. 1. By forming an electron pair (covalent) bond with another atom Non-metals typically form this type of bond with hydrogen, for example H 2 , H 2O, HCl gasor CH 4 and many metals do so too. 2. By losi ...
... proton with a charge +1 and one orbital electron. The electronic structure may be written as 1s1. 1. By forming an electron pair (covalent) bond with another atom Non-metals typically form this type of bond with hydrogen, for example H 2 , H 2O, HCl gasor CH 4 and many metals do so too. 2. By losi ...
Solid - Liquid Phase Diagram of a Binary Mixture: The Question of
... equilibrium temperature - composition diagram for co-existence of ideal solutions with these two pure solid components. One of these diagrams will assume that the carboxylic acid exists entirely as monomers in the melt, and the other will assume that it exists entirely as dimers. By analysis of thes ...
... equilibrium temperature - composition diagram for co-existence of ideal solutions with these two pure solid components. One of these diagrams will assume that the carboxylic acid exists entirely as monomers in the melt, and the other will assume that it exists entirely as dimers. By analysis of thes ...
instructor notes
... molecule. The Ar-Ag stretching frequency was found to be ~140 cm-1 and the bond length ranges from 2.56 to 2.64 Å. DFT calculations were also performed that gave predictions similar to the experimental values. The researchers concluded that the Ar-Ag interaction for these complexes is somewhere betw ...
... molecule. The Ar-Ag stretching frequency was found to be ~140 cm-1 and the bond length ranges from 2.56 to 2.64 Å. DFT calculations were also performed that gave predictions similar to the experimental values. The researchers concluded that the Ar-Ag interaction for these complexes is somewhere betw ...
Document
... Suppose the substances in the reaction are at equilibrium at 500 K. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is re-established after each of the following disturbances of the original system. Justify each answer with a brief e ...
... Suppose the substances in the reaction are at equilibrium at 500 K. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is re-established after each of the following disturbances of the original system. Justify each answer with a brief e ...
Interactive comment on “On the composition of ammonia
... Title: “On the composition of ammonia-sulfuric acid clusters during aerosol particle formation” This title is a bit misleading. The clusters that were observed in this study are ions and not electrically neutral clusters. Consider adding the word “ions” in the title to remove confusion. Line 15 pg 1 ...
... Title: “On the composition of ammonia-sulfuric acid clusters during aerosol particle formation” This title is a bit misleading. The clusters that were observed in this study are ions and not electrically neutral clusters. Consider adding the word “ions” in the title to remove confusion. Line 15 pg 1 ...
Homoleptic Two-Coordinate Silylamido Complexes of Chromium(I
... room temperature for 2 and 8, respectively, are in good agreement with the Evans’ measurements. Compound 2 exhibits a steady decrease in susceptibility upon lowering the temperature, which indicates magnetic anisotropy and temperature-independent paramagnetism (Figure 5). A small field dependence at ...
... room temperature for 2 and 8, respectively, are in good agreement with the Evans’ measurements. Compound 2 exhibits a steady decrease in susceptibility upon lowering the temperature, which indicates magnetic anisotropy and temperature-independent paramagnetism (Figure 5). A small field dependence at ...
Coordination Chemistry Reviews 272 - Didier Astruc`s Library
... donor sites nearby. When the additional donor site is adjacent to N1, coordination through N2 is possible to form a bi- or polydentate chelator (type C) [9]. Thus, for the metal chelators, five- or six-membered cycles are usually formed. Besides, bridging coordination modes with two metals coordinati ...
... donor sites nearby. When the additional donor site is adjacent to N1, coordination through N2 is possible to form a bi- or polydentate chelator (type C) [9]. Thus, for the metal chelators, five- or six-membered cycles are usually formed. Besides, bridging coordination modes with two metals coordinati ...
Chemistry 152 Laboratory Manual
... element in the Periodic Table. Valence electrons are usually represented by dots written next to the symbol for the atom. For example, H· or Na·. these are called “electron dot” formulas. In the case of Na·, the single dot represents the electron in the neutral sodium atom (23Na) has in its outermos ...
... element in the Periodic Table. Valence electrons are usually represented by dots written next to the symbol for the atom. For example, H· or Na·. these are called “electron dot” formulas. In the case of Na·, the single dot represents the electron in the neutral sodium atom (23Na) has in its outermos ...
Transition Metals and Coordination Chemistry
... Chemistry – Ch. 19 10. The compound Co(NH3)4Cl3 (233.44g/mol) has several structural isomers. A solution is made by dissolving 0.875 g of one of the isomer in 25.0 g of water. The solution freezes at -0.56 °C. (Kf = 1.86 °C kg/mol) Which of the following is the correct structural isomer? a. [Co(NH3) ...
... Chemistry – Ch. 19 10. The compound Co(NH3)4Cl3 (233.44g/mol) has several structural isomers. A solution is made by dissolving 0.875 g of one of the isomer in 25.0 g of water. The solution freezes at -0.56 °C. (Kf = 1.86 °C kg/mol) Which of the following is the correct structural isomer? a. [Co(NH3) ...
Name: Beryllium Symbol: Be Atomic number:4 Mass
... naturally occurring elements that is available for sale. Its chemistry is dominated by its tendency to lose an electron to form Be2+. As this ion is so small it is highly polarising, to the extent that its compounds are rather covalent. Its small size means that its complexes tend to be tetrahedral ...
... naturally occurring elements that is available for sale. Its chemistry is dominated by its tendency to lose an electron to form Be2+. As this ion is so small it is highly polarising, to the extent that its compounds are rather covalent. Its small size means that its complexes tend to be tetrahedral ...
Structural Features of a Sulfur-Containing Group 4 Metalla[11
... Introduction Crown ethers have a wide spread use in chemistry due to their pronounced ability for selective metal cation complexation [1]. If one of the hydrocarbyl moieties is exchanged by an electrophilic metal center then the interesting possibility arises that an internal coordination to the met ...
... Introduction Crown ethers have a wide spread use in chemistry due to their pronounced ability for selective metal cation complexation [1]. If one of the hydrocarbyl moieties is exchanged by an electrophilic metal center then the interesting possibility arises that an internal coordination to the met ...
Power Point for Equilibrium
... 1. Write an equilibrium expression for the balanced reaction. 2. Write an ICE table. Fill in the given amounts. 3. Use stoichiometry (mole ratios) on the change in concentration line. 4. Deduce the equilibrium concentrations of all species. • Usually, the initial concentration of products is zero. ( ...
... 1. Write an equilibrium expression for the balanced reaction. 2. Write an ICE table. Fill in the given amounts. 3. Use stoichiometry (mole ratios) on the change in concentration line. 4. Deduce the equilibrium concentrations of all species. • Usually, the initial concentration of products is zero. ( ...