anaplerotic (replenishing) reactions of the tca cycle - Sigma
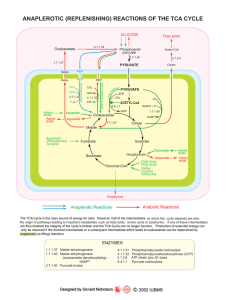
... The TCA Cycle is the main source of energy for cells. However, half of the intermediates on which the cycle depends are also the origin of pathways leading to important metabolites such as fatty acids , amino acids or porphyrins. If any of these intermediates are thus diverted the integrity of the c ...
... The TCA Cycle is the main source of energy for cells. However, half of the intermediates on which the cycle depends are also the origin of pathways leading to important metabolites such as fatty acids , amino acids or porphyrins. If any of these intermediates are thus diverted the integrity of the c ...
Microbial fermentative processes, biomass, enzymes, amino acids
... solids, dispersion of gas-liquid mixtures, aeration of liquid and heat exchange. • The stirred tank reactor is provided with a baffle and a rotating stirrer is attached either at the top or at the bottom of the bioreactor. • The typical decision variables are: type, size, location and the number of ...
... solids, dispersion of gas-liquid mixtures, aeration of liquid and heat exchange. • The stirred tank reactor is provided with a baffle and a rotating stirrer is attached either at the top or at the bottom of the bioreactor. • The typical decision variables are: type, size, location and the number of ...
Bacterial Systems for Assembly, Secretion and Targeted
... coli, use this system to carry out the invasion of host intestinal epithelial cells (Fig. 1F). A Type III system is also used by Pseudomonas syringae and other plant pathogens to deliver a set of proteins, called avirulence proteins, which induce apoptosis in the host cells (7). The type III system ...
... coli, use this system to carry out the invasion of host intestinal epithelial cells (Fig. 1F). A Type III system is also used by Pseudomonas syringae and other plant pathogens to deliver a set of proteins, called avirulence proteins, which induce apoptosis in the host cells (7). The type III system ...
r i+5
... The Ca-based model of hydrogen bonds correlates very well with the real hydrogen bonds. When “translating” the indices need to be properly shifted (by +/- 1) depending on type of secondary structure ...
... The Ca-based model of hydrogen bonds correlates very well with the real hydrogen bonds. When “translating” the indices need to be properly shifted (by +/- 1) depending on type of secondary structure ...
Amino Acids
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
Additional file 3
... that the first steps to analyze a C-type lectin with unknown functions is to perform a sequence-based analysis on its amino acid sequence to determine its (i) molecular function, (ii) biological process, and (iii) cellular location. This is followed by a structure-based analysis to further understan ...
... that the first steps to analyze a C-type lectin with unknown functions is to perform a sequence-based analysis on its amino acid sequence to determine its (i) molecular function, (ii) biological process, and (iii) cellular location. This is followed by a structure-based analysis to further understan ...
Amino Acids - Chavis Biology
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
Amino Acids - Chavis Biology
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
... group and at the opposite end is a free amino group. The ends are called the N-terminus and Cterminus. Label the N-terminus and C-terminus on your polypeptide drawn above. 15. A protein consisting of four amino acids undergoes hydrolysis. How many water molecules must be broken down and reattached t ...
MedicalBiochemistry
... The first step in analyzing a polypeptide is hydrolysis and quantitative determination of its amino acid composition. Recall that amide bonds are very resistant to hydrolysis. Hydrolysis of polypeptides requires heating in 6M HCl at 110ºC for 24-70 hr or heating 2-4M NaOH at comparable temperature a ...
... The first step in analyzing a polypeptide is hydrolysis and quantitative determination of its amino acid composition. Recall that amide bonds are very resistant to hydrolysis. Hydrolysis of polypeptides requires heating in 6M HCl at 110ºC for 24-70 hr or heating 2-4M NaOH at comparable temperature a ...
Exercises in MBV-INF 4410/9410/9410A
... (MUTYH) (546 aa), N-glycosylase/DNA lyase isoform 1a (OGG1) (345 aa) and methylCpG-binding domain protein 4 (MBD4) (580 aa). The MBD4 protein may have an E-value worse than the PSI-BLAST threshold, but is still a homolog. Give the sequences short names. b) Make a multiple sequence alignment of the f ...
... (MUTYH) (546 aa), N-glycosylase/DNA lyase isoform 1a (OGG1) (345 aa) and methylCpG-binding domain protein 4 (MBD4) (580 aa). The MBD4 protein may have an E-value worse than the PSI-BLAST threshold, but is still a homolog. Give the sequences short names. b) Make a multiple sequence alignment of the f ...
Protein Structure Prediction
... best standard sequence comparison methods such as PSI-BLAST • For the most difficult targets, the methods were able to predict 60 residues to 6.0 Å Ca RMSD, approaching comparative modelling accuracies as the similarity between proteins increased. ...
... best standard sequence comparison methods such as PSI-BLAST • For the most difficult targets, the methods were able to predict 60 residues to 6.0 Å Ca RMSD, approaching comparative modelling accuracies as the similarity between proteins increased. ...
Lecture Summary MicrobialControl(CH5)
... This chapter covers the processes available to control microbial growth. These processes are divided into two groups, physical and chemical methods of control. In my lecture notes I begin this discussion with a look at some definitions of terms, terms that we will use again when we discuss antibioti ...
... This chapter covers the processes available to control microbial growth. These processes are divided into two groups, physical and chemical methods of control. In my lecture notes I begin this discussion with a look at some definitions of terms, terms that we will use again when we discuss antibioti ...
Physical Models for Protein Folding and Drug Design
... acids, but also to design a novel class of drugs which interfere with the folding mechanism and whose inhibitor effect cannot be neutralized through mutations, as it is the case with standard drugs acting, as a rule, on the active site of enzymes. ...
... acids, but also to design a novel class of drugs which interfere with the folding mechanism and whose inhibitor effect cannot be neutralized through mutations, as it is the case with standard drugs acting, as a rule, on the active site of enzymes. ...
Functional and Structural Characterization of a Prokaryotic Peptide
... and Val-acyclovir) turned into substrates of peptide transporters with markedly improved availability (5, 6). Peptide transporters are, therefore, considered as important and potent drug delivery systems. Although functionally characterized in detail, very little is known about the structure of pept ...
... and Val-acyclovir) turned into substrates of peptide transporters with markedly improved availability (5, 6). Peptide transporters are, therefore, considered as important and potent drug delivery systems. Although functionally characterized in detail, very little is known about the structure of pept ...
Day 2 Summary
... • The 6 SPONCH elements are vital for the formation of biological macromolecules because of their chemical bonding abilities • S • P • O • N • C • H ...
... • The 6 SPONCH elements are vital for the formation of biological macromolecules because of their chemical bonding abilities • S • P • O • N • C • H ...
Chapter 1 Review Key
... 101. The recommendation to run the reaction with a substrate concentration that allows saturation of the available enzymes would lead to a stable production rate. 102. Answers may vary. Sample answer: Students’ answers should include a discussion of functional groups that distinguish the function of ...
... 101. The recommendation to run the reaction with a substrate concentration that allows saturation of the available enzymes would lead to a stable production rate. 102. Answers may vary. Sample answer: Students’ answers should include a discussion of functional groups that distinguish the function of ...
Chapter 6
... Secretion into the Periplasm • However, the presence of a signal peptide sequence does not necessarily guarantee a high rate of secretion. • The interleukin-2 gene downstream from the gene for the entire propeptide maltose-binding protein, rather than just the signal peptide, with DNA encoding the f ...
... Secretion into the Periplasm • However, the presence of a signal peptide sequence does not necessarily guarantee a high rate of secretion. • The interleukin-2 gene downstream from the gene for the entire propeptide maltose-binding protein, rather than just the signal peptide, with DNA encoding the f ...
The Plasma Membrane
... • Cholesterol keeps the fatty acid tails from sticking together, keeps the membrane stable. ...
... • Cholesterol keeps the fatty acid tails from sticking together, keeps the membrane stable. ...
Origin of Life
... Proteins and Nucleic Acid are closely linked at some fundamental level. Did proteins evolve first and produce nucleic acids, or vice-versa? Chemical Evolution of Life ...
... Proteins and Nucleic Acid are closely linked at some fundamental level. Did proteins evolve first and produce nucleic acids, or vice-versa? Chemical Evolution of Life ...
Key
... 18. VEGF is a molecule that is secreted into the blood by many human cancer cells and is a polymer of 154 amino acids. The peptide bonds of this molecule must have been made A. in the Golgi apparatus. B. on free ribosomes. C. outside of the cell. D. in or near the nucleolus. E. on the rough endoplas ...
... 18. VEGF is a molecule that is secreted into the blood by many human cancer cells and is a polymer of 154 amino acids. The peptide bonds of this molecule must have been made A. in the Golgi apparatus. B. on free ribosomes. C. outside of the cell. D. in or near the nucleolus. E. on the rough endoplas ...
Biochemistry - Austin Community College
... • Enzymes are proteins that carry out most catalysis in living organisms. • Unlike heat, enzymes are highly specific. Each enzyme typically speeds up only one or a few chemical reactions. • Unique three-dimensional shape enables an enzyme to stabilize a temporary association between substrates. • Be ...
... • Enzymes are proteins that carry out most catalysis in living organisms. • Unlike heat, enzymes are highly specific. Each enzyme typically speeds up only one or a few chemical reactions. • Unique three-dimensional shape enables an enzyme to stabilize a temporary association between substrates. • Be ...
Long Noncoding RNAs Add Another Layer to Pre
... Loci encoding long ncRNAs often overlap with or are interspersed between multiple protein-coding or noncoding genes in the genome, where they may regulate the expression of their neighbors. Moreover, long ncRNAs have been shown to act as chromatin modifiers, as transcriptional regulators that affect ...
... Loci encoding long ncRNAs often overlap with or are interspersed between multiple protein-coding or noncoding genes in the genome, where they may regulate the expression of their neighbors. Moreover, long ncRNAs have been shown to act as chromatin modifiers, as transcriptional regulators that affect ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.























