Slide 1
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
... Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. ...
biodiesel production via acid catalysis
... investigate the effect of the molar ratio of alcohol, the reaction temperature, the catalyst amount, the reaction time, and the presence of water and free fatty acids on the completeness of acid-catalyzed transesterification. Transesterification is the chemical process of converting one ester, in th ...
... investigate the effect of the molar ratio of alcohol, the reaction temperature, the catalyst amount, the reaction time, and the presence of water and free fatty acids on the completeness of acid-catalyzed transesterification. Transesterification is the chemical process of converting one ester, in th ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is found that the ammonia is 16.5% decomposed. Calculate the numerical value of the Kc for the above reaction. 3. A tank of O2 has an initial pressure of 2.00 atm. The O2 undergoes ...
... 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is found that the ammonia is 16.5% decomposed. Calculate the numerical value of the Kc for the above reaction. 3. A tank of O2 has an initial pressure of 2.00 atm. The O2 undergoes ...
高雄醫學大學九十二學年度學士後醫學系招生考試試題 科目:化學 考試
... 67. The sodium salt, NaA, of a weak acid is dissolved in water; no other substance is added. Which of the statements (to a close approximation) is true? (A) [H+] = [A-] (B) [H+] = [OH-] (C) [A-] = [OH-] (D) [HA] = [OH-] (E) none of these 68. Arrange following 0.10 M solutions from lowest to highest ...
... 67. The sodium salt, NaA, of a weak acid is dissolved in water; no other substance is added. Which of the statements (to a close approximation) is true? (A) [H+] = [A-] (B) [H+] = [OH-] (C) [A-] = [OH-] (D) [HA] = [OH-] (E) none of these 68. Arrange following 0.10 M solutions from lowest to highest ...
45.1 Inter-conversions between the functional groups
... Distil the product mixture and collect the liquid that boils between 110°C and 114°C. The distillate obtained is an aqueous solution of ethanoic acid and it has a strong smell of vinegar. To obtain pure ethanoic acid, the aqueous solution of ethanoic acid is redistilled. The liquid that boils betwe ...
... Distil the product mixture and collect the liquid that boils between 110°C and 114°C. The distillate obtained is an aqueous solution of ethanoic acid and it has a strong smell of vinegar. To obtain pure ethanoic acid, the aqueous solution of ethanoic acid is redistilled. The liquid that boils betwe ...
Alcohols General formula R-OH hydroxyl group Nomenclature
... C Cl + H2 O CH3 i.e. SN 1 reaction Clnotice that another reaction of carbocations is loss of H+ to yield an alkene. In this example, the nucleophile Clis in large excess and so the formation of the alkyl halide is favored. ...
... C Cl + H2 O CH3 i.e. SN 1 reaction Clnotice that another reaction of carbocations is loss of H+ to yield an alkene. In this example, the nucleophile Clis in large excess and so the formation of the alkyl halide is favored. ...
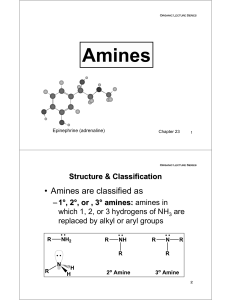
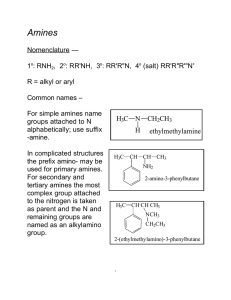
Amines
... – pyridine is a weaker base than heterocyclic aliphatic amines because the free electron pair on N lies in an sp2 hybrid orbital (33% s character) and is held more tightly to the nucleus than the free electron pair on N in an sp3 hybrid orbital (25% s character) ...
... – pyridine is a weaker base than heterocyclic aliphatic amines because the free electron pair on N lies in an sp2 hybrid orbital (33% s character) and is held more tightly to the nucleus than the free electron pair on N in an sp3 hybrid orbital (25% s character) ...
EXPERIMENT 6 (Organic Chemistry II) Pahlavan/Cherif
... Aldehydes and ketones share the carbonyl functional group which features carbon doubly bonded to oxygen. In the case of ketones there are two carbon atoms bonded to the carbonyl carbon and no hydrogens. In the case of aldehydes there is at least one hydrogen bonded to the carbonyl carbon, the other ...
... Aldehydes and ketones share the carbonyl functional group which features carbon doubly bonded to oxygen. In the case of ketones there are two carbon atoms bonded to the carbonyl carbon and no hydrogens. In the case of aldehydes there is at least one hydrogen bonded to the carbonyl carbon, the other ...
1 - University of Missouri
... 4. Analyze the IR spectrum of your product, filling in the table below. You should utilize your course textbook (pp. 764-766), if you need assistance. Please note that the frequency given below should be exact (one number), not a range. Use the frequency of the center of the band at its strongest po ...
... 4. Analyze the IR spectrum of your product, filling in the table below. You should utilize your course textbook (pp. 764-766), if you need assistance. Please note that the frequency given below should be exact (one number), not a range. Use the frequency of the center of the band at its strongest po ...
Structure and Bonding
... each end of the double bond. It is also called a catalytic hydrogenation or a reduction reaction. The catalyst is crucial since the reaction will not take place at room temperature in its absence. ...
... each end of the double bond. It is also called a catalytic hydrogenation or a reduction reaction. The catalyst is crucial since the reaction will not take place at room temperature in its absence. ...
barbituates
... Barbituric acid was first created in 1864 by a German scientist named Adolf von Baeyer. It was a combination of urea from animals and malonic acid from apples. Its first derivative utilized as medicine was used to put dogs to sleep but was soon produced by Bayer as a sleep aid in 1903 called Veronal ...
... Barbituric acid was first created in 1864 by a German scientist named Adolf von Baeyer. It was a combination of urea from animals and malonic acid from apples. Its first derivative utilized as medicine was used to put dogs to sleep but was soon produced by Bayer as a sleep aid in 1903 called Veronal ...
Alcohols I Reading: Wade chapter 10, sections 10-1- 10
... 1. Organometallic reagents in the synthesis of alcohols Organometallic compounds contain a highly polarized covalent bond between carbon and a metal atom (C–M, M=Li, Na, K, Mg). The C–M bond is polarized so that most of the electron density resides on carbon, since it is the more electronegative ato ...
... 1. Organometallic reagents in the synthesis of alcohols Organometallic compounds contain a highly polarized covalent bond between carbon and a metal atom (C–M, M=Li, Na, K, Mg). The C–M bond is polarized so that most of the electron density resides on carbon, since it is the more electronegative ato ...
102 Lecture Ch15
... • Aldehydes and ketones can be flammable and/or toxic, though generally not highly so • They usually have strong odors, and are often used as flavorings or scents ...
... • Aldehydes and ketones can be flammable and/or toxic, though generally not highly so • They usually have strong odors, and are often used as flavorings or scents ...
Exam 1 Review Sheet Chapter 15 Chemistry 110b
... Amides and lactams: methods for the synthesis of amides . Hydrolysis of amides: know the acid and base mechanisms, compare these hydrolyses with the ester case in terms of ease of hydrolysis. Lactams--nomenclature, properties, occurrence, and reactions. [10e, 804-812; 11e, 796-802] ...
... Amides and lactams: methods for the synthesis of amides . Hydrolysis of amides: know the acid and base mechanisms, compare these hydrolyses with the ester case in terms of ease of hydrolysis. Lactams--nomenclature, properties, occurrence, and reactions. [10e, 804-812; 11e, 796-802] ...
Catalysis in Biodiesel Production by Transesterification
... the protonated catalyst. The second step is the nucleophilic attack of the alkoxide at the carbonyl group of the triglyceride generating a tetrahedral intermediate24-26. The third step involves the formation of the alkyl ester and the corresponding anion of diglyceride. The final step involves depro ...
... the protonated catalyst. The second step is the nucleophilic attack of the alkoxide at the carbonyl group of the triglyceride generating a tetrahedral intermediate24-26. The third step involves the formation of the alkyl ester and the corresponding anion of diglyceride. The final step involves depro ...
CARBONYL COMPOUNDS
... The silver mirror test is the better alternative as it works with all aldehydes. Ketones do not react with Tollens’ Reagent or Fehling’s Solution. ...
... The silver mirror test is the better alternative as it works with all aldehydes. Ketones do not react with Tollens’ Reagent or Fehling’s Solution. ...
Chemical Reactions
... The cation of the ionic compound on the reactant side is neutralized on the product side as it becomes an element. If you follow these alterations, it will be easy to identify this as a redox reaction and visualize the electron transfer. The simplest way to follow the alteration is to assign oxidati ...
... The cation of the ionic compound on the reactant side is neutralized on the product side as it becomes an element. If you follow these alterations, it will be easy to identify this as a redox reaction and visualize the electron transfer. The simplest way to follow the alteration is to assign oxidati ...
final1-final_report
... ligands. Since chiral bridgehead or ring substituents are in close proximity to the Al/M active metal centres, both are likely to have an effect on chiral transmission to a pro-chiral alkene substrate such as terminal alkenes of the type RCH=CH2. We have explored two obvious ways to introduce chiral ...
... ligands. Since chiral bridgehead or ring substituents are in close proximity to the Al/M active metal centres, both are likely to have an effect on chiral transmission to a pro-chiral alkene substrate such as terminal alkenes of the type RCH=CH2. We have explored two obvious ways to introduce chiral ...
Year 12 Unit 1b - Moulsham High School
... Describe the appearance of the mixture after compound C is boiled with Benedict’s solution. ...
... Describe the appearance of the mixture after compound C is boiled with Benedict’s solution. ...
CH 2 OH
... hydrogen atoms bonded to other atoms Organic molecules are a diverse group Four types of organic molecules (biomolecules) exist in organisms: ...
... hydrogen atoms bonded to other atoms Organic molecules are a diverse group Four types of organic molecules (biomolecules) exist in organisms: ...
Chapter 1 Chemical Bonding and Chemical Structure
... • A phenyl group can be represented a few different ways ...
... • A phenyl group can be represented a few different ways ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.