A Mild and Convenient Conversion of Ketones to the Corresponding
... to incorporate deuterium regiospecifically by using deuterium oxide as the source of deuterium.’O The purpose of this study was to investigate reducing agents which are as versatile as catecholborane but which can be more readily prepared. We report that bis(benzoyloxy)borane, I,l1J2effectively redu ...
... to incorporate deuterium regiospecifically by using deuterium oxide as the source of deuterium.’O The purpose of this study was to investigate reducing agents which are as versatile as catecholborane but which can be more readily prepared. We report that bis(benzoyloxy)borane, I,l1J2effectively redu ...
Bioorganic chemistry_1
... A. Derivative hydrocarbons, in molecules which one or more hydrogen atoms substituted into a hydroxy-group. B. Compounds in which one or more hydrogen atoms substituted into alkyl radicals. C. Compounds molecule which consist of a lot structural links. D. Cyclic compounds in which cycle have Oxygen. ...
... A. Derivative hydrocarbons, in molecules which one or more hydrogen atoms substituted into a hydroxy-group. B. Compounds in which one or more hydrogen atoms substituted into alkyl radicals. C. Compounds molecule which consist of a lot structural links. D. Cyclic compounds in which cycle have Oxygen. ...
Experiment 7: Acidity of Alcohols Williamson Ether Synthesis of
... compound in your notebook. What effect does an electron-donating group like a methyl (-CH3) or an alkyl group have on the resulting alkoxide produced from the reaction with sodium? Use pH paper to measure the approximate pH of each alcohol solution after the sodium has reacted. Record the pH's. Add ...
... compound in your notebook. What effect does an electron-donating group like a methyl (-CH3) or an alkyl group have on the resulting alkoxide produced from the reaction with sodium? Use pH paper to measure the approximate pH of each alcohol solution after the sodium has reacted. Record the pH's. Add ...
Synthetic route to novel asymmetric tetradentate ligands
... adjacent positions of the coordination sphere may be vacant or filled with labile ligands. The different hybridization mode for the amino (sp3) and imino (sp2) groups may favour the tetracoordination of the organic ligand outside from the equatorial plane of the metal ion. In this case, two of the c ...
... adjacent positions of the coordination sphere may be vacant or filled with labile ligands. The different hybridization mode for the amino (sp3) and imino (sp2) groups may favour the tetracoordination of the organic ligand outside from the equatorial plane of the metal ion. In this case, two of the c ...
Exames anteriores a 1994
... Upon heating of a mixture of A and fluorine (molar ratio 1:9, high pressure) to 900 °C three compounds (B, C and D) are formed. All three products are crystalline solids with melting points below 150 °C. The fluorine content of C is found to be 36.7% and that of D 46.5% (by weight). When B is treate ...
... Upon heating of a mixture of A and fluorine (molar ratio 1:9, high pressure) to 900 °C three compounds (B, C and D) are formed. All three products are crystalline solids with melting points below 150 °C. The fluorine content of C is found to be 36.7% and that of D 46.5% (by weight). When B is treate ...
evans enolate alkylation
... Enantioselective Synthesis Preparation of Enantiomerically enriched Compounds This is critically important because the two enantiomers of the same compounds often/usually have very different properties when in come to biological activity. There are a number of different types of approaches to enanti ...
... Enantioselective Synthesis Preparation of Enantiomerically enriched Compounds This is critically important because the two enantiomers of the same compounds often/usually have very different properties when in come to biological activity. There are a number of different types of approaches to enanti ...
Asymmetric Glycine Enolate Aldol Reactions
... Asymmetric Glycine Enolate Aldol Reactions: Synthesis of Cyclosporine's Unusual Amino Acid, MeBmt David A. Evans* and Ann E. Weber2 Contribution from the Department of Chemistry, Haruard University, Cambridge, Massachusetts 02138. Received May I , 1986 ...
... Asymmetric Glycine Enolate Aldol Reactions: Synthesis of Cyclosporine's Unusual Amino Acid, MeBmt David A. Evans* and Ann E. Weber2 Contribution from the Department of Chemistry, Haruard University, Cambridge, Massachusetts 02138. Received May I , 1986 ...
T10 SL - MsReenChemistry
... Explain, using equations, the following steps in the free-radical mechanism of the reaction of methane with chlorine. ...
... Explain, using equations, the following steps in the free-radical mechanism of the reaction of methane with chlorine. ...
Organic Synthesis - National Open University of Nigeria
... On oxidation with peroxy-acids, ketones are converted into esters or lactones. This reaction was discovered in 1899 by Baeyer and Villiger. Better yields are obtained with organic peroxy-acids such as perbenzoic acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most rea ...
... On oxidation with peroxy-acids, ketones are converted into esters or lactones. This reaction was discovered in 1899 by Baeyer and Villiger. Better yields are obtained with organic peroxy-acids such as perbenzoic acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most rea ...
A-level Chemistry Question paper Unit 04 - Kinetics, Equilibria
... an inert solvent in the presence of a small amount of concentrated sulfuric acid. The equilibrium mixture formed contained 1.80 mol of DEM in a total volume, V dm3, of solution. Calculate the amount (in moles) of A, of ethanol and of water in this equilibrium ...
... an inert solvent in the presence of a small amount of concentrated sulfuric acid. The equilibrium mixture formed contained 1.80 mol of DEM in a total volume, V dm3, of solution. Calculate the amount (in moles) of A, of ethanol and of water in this equilibrium ...
Practice Problem
... dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes ...
... dialkylcopper (Gilman reagents) 3. Lithium dialkylcopper reagents react with alkyl halides to give alkanes ...
chapter 8-carboxyl compounds
... • Hexanedioic acid, HOOC(CH2)4COOH: - manufacture of nylon 6,6 • Benzoic acid and sodium benzoate: - as preservatives in foodstuff. • 2-hydroxybenzoic acid: ...
... • Hexanedioic acid, HOOC(CH2)4COOH: - manufacture of nylon 6,6 • Benzoic acid and sodium benzoate: - as preservatives in foodstuff. • 2-hydroxybenzoic acid: ...
Substrate scope of the re-engineered enzyme, FucO D93
... ”A catalyst is a substance that accelerates a reaction but undergoes no net chemical change”. This is done by lowering the activation energy of a reaction, either by stabilizing intermediates or by changing the reaction pathways to avoid slow rate-limiting steps, see Fig 2.1. [1] Catalysis is usuall ...
... ”A catalyst is a substance that accelerates a reaction but undergoes no net chemical change”. This is done by lowering the activation energy of a reaction, either by stabilizing intermediates or by changing the reaction pathways to avoid slow rate-limiting steps, see Fig 2.1. [1] Catalysis is usuall ...
Student Learning Outcomes (broken down by chapter…basically the
... Predict the major products of the dehydration of alcohols and describe the relative rates at which alcohols undergo dehydration. Predict the products of the reactions of alcohols with oxidizing reagents. Propose mechanisms for the dehydration reactions of alcohols. Predict the major products of the ...
... Predict the major products of the dehydration of alcohols and describe the relative rates at which alcohols undergo dehydration. Predict the products of the reactions of alcohols with oxidizing reagents. Propose mechanisms for the dehydration reactions of alcohols. Predict the major products of the ...
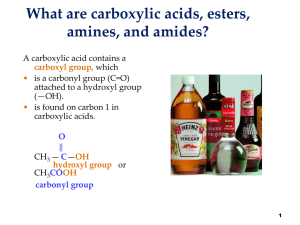
Esters amines and amides
... nonpolar fats and oils, and a polar end that dissolves in water. • forms groups of soap ...
... nonpolar fats and oils, and a polar end that dissolves in water. • forms groups of soap ...
Supplementary material - Royal Society of Chemistry
... On addition of another two equivalents of the sodium salt of diethyl acetamidomalonate to the reaction mixture, the allylic alcohol (18) reacted to give a new product as adjudged by TLC. The structure of 16 determined by X-ray crystallography shows that the epoxide forms exclusively on the opposite ...
... On addition of another two equivalents of the sodium salt of diethyl acetamidomalonate to the reaction mixture, the allylic alcohol (18) reacted to give a new product as adjudged by TLC. The structure of 16 determined by X-ray crystallography shows that the epoxide forms exclusively on the opposite ...
Ethers and Epoxides - Delaware State University
... Carbon NMR: C’s in ethers exhibit a downfield shift to 50 to 80 ...
... Carbon NMR: C’s in ethers exhibit a downfield shift to 50 to 80 ...
Chapter in Zumdahl: Chapter #12 Kinetics (2
... know the solubility rules and be able to apply them to given substances. write a Ksp expression for a slightly soluble salt. calculate the numerical value of the Ksp, given solubility data. calculate the solubility of a substance, given the Ksp. will be given a Ksp, predict the relative solubility’s ...
... know the solubility rules and be able to apply them to given substances. write a Ksp expression for a slightly soluble salt. calculate the numerical value of the Ksp, given solubility data. calculate the solubility of a substance, given the Ksp. will be given a Ksp, predict the relative solubility’s ...
Paper
... 0.86) and isopropyl myristate (Rf 0.70) being used as reference. The visualization of the chromatographic traces was carried out via spraying by a 10 % solution of sulfuric acid in ethanol and subsequent annealing the plates. It was revealed that the cyclohexane fractions of liquid and solid alkylat ...
... 0.86) and isopropyl myristate (Rf 0.70) being used as reference. The visualization of the chromatographic traces was carried out via spraying by a 10 % solution of sulfuric acid in ethanol and subsequent annealing the plates. It was revealed that the cyclohexane fractions of liquid and solid alkylat ...
Lab 9
... Water, alcohols and ethers are similar in that they all contain a single oxygen atom. Figure 1 shows the structural relationships among them. ...
... Water, alcohols and ethers are similar in that they all contain a single oxygen atom. Figure 1 shows the structural relationships among them. ...
12.1 Alcohols: Structure and Physical Properties
... • These changes are easily detected in inorganic systems with formation of charged ions • In organic systems it is often difficult to determine whether oxidation or reduction has taken place as there might be no change in charge ...
... • These changes are easily detected in inorganic systems with formation of charged ions • In organic systems it is often difficult to determine whether oxidation or reduction has taken place as there might be no change in charge ...
Some more basic organic (more naming, reactions, polymers)
... 1,3,5-hexatriene This is not the case! 1,3,5-hexatriene is fairly reactive with a variety of reagents (e.g. HBr, Cl2, etc. in the dark). These reagents react only slowly with benzene. ...
... 1,3,5-hexatriene This is not the case! 1,3,5-hexatriene is fairly reactive with a variety of reagents (e.g. HBr, Cl2, etc. in the dark). These reagents react only slowly with benzene. ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.