Naming Organic Compounds
... hydrogen on nitrogen, it can react with an organic acid to produce an amide and water R – C=O-OH + R’NH2 RC=ONHR’ + H2O ...
... hydrogen on nitrogen, it can react with an organic acid to produce an amide and water R – C=O-OH + R’NH2 RC=ONHR’ + H2O ...
National German Competition
... q) Write down the equation of the reaction of compound 1 with lithium dimethylcuprate and water. Give the complete names of the alcohols. Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. ...
... q) Write down the equation of the reaction of compound 1 with lithium dimethylcuprate and water. Give the complete names of the alcohols. Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. ...
Go FIGure
... athletic injuries (▶ Figure 13.5). The packs consist of a pouch of water and the solid salt sealed off from the water—MgSO41s2 for hot packs and NH4NO31s2 for cold packs. When the pack is squeezed, the seal separating the solid from the water is broken and a solution forms, either increasing or decr ...
... athletic injuries (▶ Figure 13.5). The packs consist of a pouch of water and the solid salt sealed off from the water—MgSO41s2 for hot packs and NH4NO31s2 for cold packs. When the pack is squeezed, the seal separating the solid from the water is broken and a solution forms, either increasing or decr ...
Chemistry - Edexcel
... 17 The amino acid alanine, H2NCH(CH3)COOH, exists as a solid at room temperature. The most important reason for this is that it A exists as a zwitterion. B forms hydrogen bonds. C is amphoteric. D has strong London forces. ...
... 17 The amino acid alanine, H2NCH(CH3)COOH, exists as a solid at room temperature. The most important reason for this is that it A exists as a zwitterion. B forms hydrogen bonds. C is amphoteric. D has strong London forces. ...
Krystal-Klear-info-Sheet-Agriculture
... a metal cation and a ligand to form a chelating complex: A good stability constant is one in which the bond is strong enough to hold the metal in solution but not so strong that it doesn’t release the metal when applied. Number of ligands: This refers to the ligands or “legs” that bond the metal to ...
... a metal cation and a ligand to form a chelating complex: A good stability constant is one in which the bond is strong enough to hold the metal in solution but not so strong that it doesn’t release the metal when applied. Number of ligands: This refers to the ligands or “legs” that bond the metal to ...
4 Investigation of substituent effect on
... complexes [Cr(CO)5{C(X)R}] depends mainly on the electronic characteristics of the carbene substituents X and R, which seem to have remarkable control on the electrophilicity of the complex.30 Specifically, it has been proposed that the -bond character of a metal carbene can be better represented by ...
... complexes [Cr(CO)5{C(X)R}] depends mainly on the electronic characteristics of the carbene substituents X and R, which seem to have remarkable control on the electrophilicity of the complex.30 Specifically, it has been proposed that the -bond character of a metal carbene can be better represented by ...
aminobromination.alk..
... was set to 5 keV. Column chromatography was performed on silica gel 60 (Baker, 200– 400 mesh). All commercially available starting materials were purchased from Fluka and used without additional purification. 4.1.1. Preparation of t-butyl N,N-dibromocarbamate (BBC, 3). Bromine (35.16 g, 0.22 mol) wa ...
... was set to 5 keV. Column chromatography was performed on silica gel 60 (Baker, 200– 400 mesh). All commercially available starting materials were purchased from Fluka and used without additional purification. 4.1.1. Preparation of t-butyl N,N-dibromocarbamate (BBC, 3). Bromine (35.16 g, 0.22 mol) wa ...
Late Transition Metal Amido Complexes: Electronic
... catalytic C-N bond forming reactions. The introduction of these precious metal catalysts in the homogeneous olefin hydroamination represents an interesting alternative to group 4 and lanthanoid based catalysts due to the generally higher functional group tolerance.27 Two mechanisms are conceivable f ...
... catalytic C-N bond forming reactions. The introduction of these precious metal catalysts in the homogeneous olefin hydroamination represents an interesting alternative to group 4 and lanthanoid based catalysts due to the generally higher functional group tolerance.27 Two mechanisms are conceivable f ...
CH 3
... The Carbon-Carbon bond is 1.2Ao shorter than C=C, which is 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
... The Carbon-Carbon bond is 1.2Ao shorter than C=C, which is 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
CH 3
... The Carbon-Carbon bond is 1.2Ao shorter than C=C, which is 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
... The Carbon-Carbon bond is 1.2Ao shorter than C=C, which is 1.3Ao. C-H bond is also shorter than ethene, which is shorter than ethane, because in ethyne it is overlap between an sp orbital and a s-orbital of H to give the s-bond. The23/05/2017 bonding electrons reside closer to the C-nucleus, and so ...
Critical Analysis of the Melanogenic Pathway in Insects and Higher
... Melanins are a group of widely distributed phenolic biopolymers arising from the amino acid, tyrosine and related phenolic compounds [1–3]. They are responsible for the skin, hair (or coat or feather), and eye color in animals. Plants as well as fungi and even some bacteria synthesize melanin not on ...
... Melanins are a group of widely distributed phenolic biopolymers arising from the amino acid, tyrosine and related phenolic compounds [1–3]. They are responsible for the skin, hair (or coat or feather), and eye color in animals. Plants as well as fungi and even some bacteria synthesize melanin not on ...
Chapter 6 Addition Reactions to Alkenes
... Boron hydride addition to alkenes followed by oxidation will give alcohols in which the – OH group is attached to the less substituted carbon, the opposite regiochemistry to that observed with the simple acid catalyzed hydration of alkenes. H CH3 C C H CH3 ...
... Boron hydride addition to alkenes followed by oxidation will give alcohols in which the – OH group is attached to the less substituted carbon, the opposite regiochemistry to that observed with the simple acid catalyzed hydration of alkenes. H CH3 C C H CH3 ...
Article VII
... This paper presents the application of the hybrid method EV1OMM17 to this problem. This method, mixing quantum mechanics (QM) and molecular mechanics (MM) descriptions for different parts of the same system, has already been proved successful in a number of examples,I8~20 including a case with compl ...
... This paper presents the application of the hybrid method EV1OMM17 to this problem. This method, mixing quantum mechanics (QM) and molecular mechanics (MM) descriptions for different parts of the same system, has already been proved successful in a number of examples,I8~20 including a case with compl ...
CHEMISTRY 211 FINAL EXAM Wed., December 4, 2002 Name
... product (B) {optical rotation +20°}. However, treatment of (A) with CH3OH gave 2 products, (C) and (D) {optical rotations +37° and -37°, respectively}. Draw products (B), (C) and (D) and explain briefly, but clearly, what type of reaction is taking place in each case and why this would lead to the ...
... product (B) {optical rotation +20°}. However, treatment of (A) with CH3OH gave 2 products, (C) and (D) {optical rotations +37° and -37°, respectively}. Draw products (B), (C) and (D) and explain briefly, but clearly, what type of reaction is taking place in each case and why this would lead to the ...
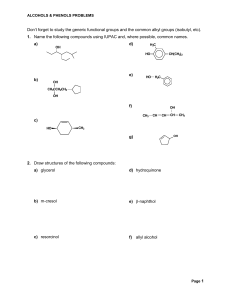
Don`t forget to study the generic functional groups and the common
... CH3 CH3 the type of reaction H3C ...
... CH3 CH3 the type of reaction H3C ...
Ozone decomposition
... above 105 °C. The gaseous ozone is characterized by different times of half-life, depending on the temperature (Table 1). The ozone structure is resonance stabilized, which is one of the reasons for its resistance against decomposition at low temperatures (Figure 2). In most reactions with inorganic ...
... above 105 °C. The gaseous ozone is characterized by different times of half-life, depending on the temperature (Table 1). The ozone structure is resonance stabilized, which is one of the reasons for its resistance against decomposition at low temperatures (Figure 2). In most reactions with inorganic ...
Davin, Thomas J. (2009) Computational chemistry of organometallic
... This thesis presents computational investigations of problems related to redox processes and structural rearrangement in inorganic systems. Density functional theory has been used to gain insight into the origin and nature of such reactions. The work presented concerns two main topics: hydrogenase-l ...
... This thesis presents computational investigations of problems related to redox processes and structural rearrangement in inorganic systems. Density functional theory has been used to gain insight into the origin and nature of such reactions. The work presented concerns two main topics: hydrogenase-l ...
AQA A-level Chemistry
... specific heat capacity of sea water is greater than that of the land and so more heat energy is needed to heat up the sea by the same amount as the land and so it takes longer. It also takes longer to cool down. ...
... specific heat capacity of sea water is greater than that of the land and so more heat energy is needed to heat up the sea by the same amount as the land and so it takes longer. It also takes longer to cool down. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.