Molecular Encapsulation - Colin Nuckolls
... the role of solvent in the formation of the capsules cannot be ignored. The medium must not disrupt the interactions that hold the components of the capsule together. Capsules constructed through metal ± ligand interactions are typically disrupted by strongly ligating solvents, while they may remain ...
... the role of solvent in the formation of the capsules cannot be ignored. The medium must not disrupt the interactions that hold the components of the capsule together. Capsules constructed through metal ± ligand interactions are typically disrupted by strongly ligating solvents, while they may remain ...
Synthetic chemistry with nitrous oxide
... a heterocubane structure (Scheme 3b).33 A similar reaction was observed for the pentamethylcyclopentadienyl complex Cp*2Cr.34 Oxygen atom transfer was also demonstrated for cyclopentadienyl complexes of vanadium,35 tantalum,36 zirconium,37 and hafnium.38 Oxygen atom transfer reactions are not restri ...
... a heterocubane structure (Scheme 3b).33 A similar reaction was observed for the pentamethylcyclopentadienyl complex Cp*2Cr.34 Oxygen atom transfer was also demonstrated for cyclopentadienyl complexes of vanadium,35 tantalum,36 zirconium,37 and hafnium.38 Oxygen atom transfer reactions are not restri ...
CH 3 - bYTEBoss
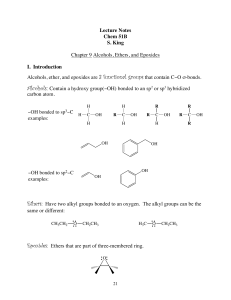
... covalently bonded to an aliphatic carbon atom. • The first four halogens— fluorine, chlorine, bromine, and iodine—are found in many organic compounds. ...
... covalently bonded to an aliphatic carbon atom. • The first four halogens— fluorine, chlorine, bromine, and iodine—are found in many organic compounds. ...
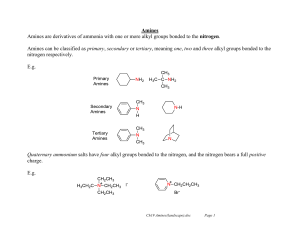
226 amines lec
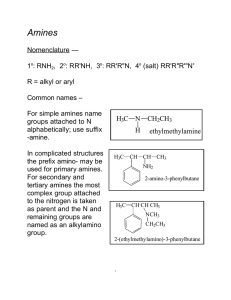
... Vinyl and aryl halides usually do not react. A severe limitation to ammonolysis in many cases is generation of other classes of amines — ...
... Vinyl and aryl halides usually do not react. A severe limitation to ammonolysis in many cases is generation of other classes of amines — ...
File
... In other words, a ________ value for Keq means that at equilibrium, there is _____________ and very little reactant left. The larger the value for Keq the closer to completion the reaction is at equilibrium. (NOTE: "Completion" means reactants have been completely converted to products.) A very smal ...
... In other words, a ________ value for Keq means that at equilibrium, there is _____________ and very little reactant left. The larger the value for Keq the closer to completion the reaction is at equilibrium. (NOTE: "Completion" means reactants have been completely converted to products.) A very smal ...
Complexometric Reactions and Titrations
... A ligand is called a monodentate if it donates a single pair of electrons (like :NH3) while a bidentate ligand (like ethylenediamine, :NH2CH2CH2H2N:) donates two pairs of electrons. Ethylenediaminetetraacetic acid (EDTA) is a hexadentate ligand. The ligand can be as simple as ammonia which forms a ...
... A ligand is called a monodentate if it donates a single pair of electrons (like :NH3) while a bidentate ligand (like ethylenediamine, :NH2CH2CH2H2N:) donates two pairs of electrons. Ethylenediaminetetraacetic acid (EDTA) is a hexadentate ligand. The ligand can be as simple as ammonia which forms a ...
Immobilization and stability studies of a lipase from
... fine chemicals. Many lipases are only moderately stable at high temperature and pHs, that can influence their usefulness in some interesting reactions. Using lipases from thermophilic microorganisms, whose resistance to drastic conditions has been developed by nature, can solve this problem. At pres ...
... fine chemicals. Many lipases are only moderately stable at high temperature and pHs, that can influence their usefulness in some interesting reactions. Using lipases from thermophilic microorganisms, whose resistance to drastic conditions has been developed by nature, can solve this problem. At pres ...
Organometallic C-H Bond Activation: An Introduction
... outer-sphere oxidant, is also consistent with the experimental data and should be considered for this reaction (1a). Others have also followed up on Shilov’s original observations, and numerous systems involving oxidation of alkanes by metal cations in acidic solution have been reported (18). While ...
... outer-sphere oxidant, is also consistent with the experimental data and should be considered for this reaction (1a). Others have also followed up on Shilov’s original observations, and numerous systems involving oxidation of alkanes by metal cations in acidic solution have been reported (18). While ...
Chapter 14 Aldehydes, Ketones, and Chiral Molecules
... Carbonyl Group in Aldehydes and Ketones A carbonyl group • In an aldehyde is attached to at least one H atom. • In a ketone is attached to two carbon groups. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
... Carbonyl Group in Aldehydes and Ketones A carbonyl group • In an aldehyde is attached to at least one H atom. • In a ketone is attached to two carbon groups. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings ...
Organic handouts - Moore Chemistry
... These hydrocarbons exist in ring structures with general formula CnH2n. There is considerable bond strain for 3 and 4 carbon rings. 5 and 6 carbon rings have much less bond strain. They can buckle their structures to achieve more stability. You name them just as alkanes but add the prefix CYCLOAlken ...
... These hydrocarbons exist in ring structures with general formula CnH2n. There is considerable bond strain for 3 and 4 carbon rings. 5 and 6 carbon rings have much less bond strain. They can buckle their structures to achieve more stability. You name them just as alkanes but add the prefix CYCLOAlken ...
Document
... • Side chains are also called “side branches” or “alkyl groups”. Their names end in -yl. ...
... • Side chains are also called “side branches” or “alkyl groups”. Their names end in -yl. ...
Document
... • Side chains are also called “side branches” or “alkyl groups”. Their names end in -yl. ...
... • Side chains are also called “side branches” or “alkyl groups”. Their names end in -yl. ...
isomeria geometrica
... • Different molecules (enantiomers) must have different names. • Usually only one enantiomer will be biologically active. • Configuration around the chiral carbon is specified with (R) and (S). ...
... • Different molecules (enantiomers) must have different names. • Usually only one enantiomer will be biologically active. • Configuration around the chiral carbon is specified with (R) and (S). ...
Enyne Metathesis (Enyne Bond Reorganization)
... bling, so the mechanism must have occurred through an intramolecular process. Migration of the carbon-13 label can be explained by the mechanism formulated in Scheme 5. Cyclopalladation gives 16, which undergoes reductive elimination to yield η2-complexed cyclobutene 17. When R ) CO2Me, the cyclobut ...
... bling, so the mechanism must have occurred through an intramolecular process. Migration of the carbon-13 label can be explained by the mechanism formulated in Scheme 5. Cyclopalladation gives 16, which undergoes reductive elimination to yield η2-complexed cyclobutene 17. When R ) CO2Me, the cyclobut ...
Full text (no figures)
... 22. The method of claim 21 wherein the binary or tertiary alumina is a .gamma.-alumina. 23. The method of claim 21 wherein the heating step is performed at temperatures ranging from 325.degree. C. to 600.degree. C. 24. The method of claim 21 wherein the heating step is performed at temperatures rang ...
... 22. The method of claim 21 wherein the binary or tertiary alumina is a .gamma.-alumina. 23. The method of claim 21 wherein the heating step is performed at temperatures ranging from 325.degree. C. to 600.degree. C. 24. The method of claim 21 wherein the heating step is performed at temperatures rang ...
Hydrogen Fuel—An Economically Viable Future for
... hydrogen from a pressurized mixture and release it when depressurized, via carbon dioxide absorption, or with membranes that are selectively permeable to hydrogen. Reforming is approximately 90% efficient, meaning that at the higher heating value (see Table 1), 90% of the energy content of the origi ...
... hydrogen from a pressurized mixture and release it when depressurized, via carbon dioxide absorption, or with membranes that are selectively permeable to hydrogen. Reforming is approximately 90% efficient, meaning that at the higher heating value (see Table 1), 90% of the energy content of the origi ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.