catalysis lecture
... 5. Oxidative Addition & Reductive Elimination Oxidative Addition of a molecule AX to a complex: • dissociation of the A—X bond • coordination of the two fragments to the metal center ...
... 5. Oxidative Addition & Reductive Elimination Oxidative Addition of a molecule AX to a complex: • dissociation of the A—X bond • coordination of the two fragments to the metal center ...
Carboxylic Acids - BSAK Chemistry weebly
... • The first stage (the addition stage of the reaction) involves a nucleophilic attack on the fairly positive carbon atom by one of the lone pairs on the oxygen of a water molecule. ...
... • The first stage (the addition stage of the reaction) involves a nucleophilic attack on the fairly positive carbon atom by one of the lone pairs on the oxygen of a water molecule. ...
Cross-coupling reactions of organoborons with organic halides
... in the presence of wide variety of functional groups. Several organometallic reagents are used for analogous cross-coupling reactions but organoboron compounds are interesting reagents because they are thermally stable, inert to water and oxygen and thus easy to handle. In addition, inorganic by-pro ...
... in the presence of wide variety of functional groups. Several organometallic reagents are used for analogous cross-coupling reactions but organoboron compounds are interesting reagents because they are thermally stable, inert to water and oxygen and thus easy to handle. In addition, inorganic by-pro ...
Chapter 7: Alkene reactions
... Example: Compound A has the formula C10H16. On catalytic hydrogenation over palladium (H2, Pd) it reacts with only one molar equivalent of H2. Compound A also undergoes reaction with ozone (O3), followed by zinc treatment (Zn, H3O+) to yield a symmetrical diketone, B which has formula (C10H16O2). Pr ...
... Example: Compound A has the formula C10H16. On catalytic hydrogenation over palladium (H2, Pd) it reacts with only one molar equivalent of H2. Compound A also undergoes reaction with ozone (O3), followed by zinc treatment (Zn, H3O+) to yield a symmetrical diketone, B which has formula (C10H16O2). Pr ...
Theoretical Competition - Austrian Chemistry Olympiad
... 2.2. Give the formula and the name of complex K1. 2.3. Draw the occupation of the d-orbitals for K1 and verify it by comparing the calculated and the measured magnetic moment. 2.4. Calculate the ligand energy splitting ∆ (in kJ/mol) for K1. 2.5. In case of the same central ion and the ligands H2O, C ...
... 2.2. Give the formula and the name of complex K1. 2.3. Draw the occupation of the d-orbitals for K1 and verify it by comparing the calculated and the measured magnetic moment. 2.4. Calculate the ligand energy splitting ∆ (in kJ/mol) for K1. 2.5. In case of the same central ion and the ligands H2O, C ...
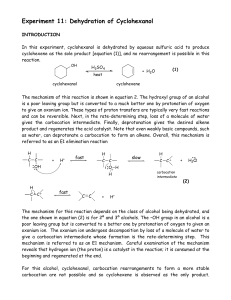
Dehydration of Cyclohexanol
... The mechanism for this reaction depends on the class of alcohol being dehydrated, and the one shown in equation (2) is for 2º and 3º alcohols. The –OH group in an alcohol is a poor leaving group but is converted to a better one by protonation of oxygen to given an oxonium ion. The oxonium ion underg ...
... The mechanism for this reaction depends on the class of alcohol being dehydrated, and the one shown in equation (2) is for 2º and 3º alcohols. The –OH group in an alcohol is a poor leaving group but is converted to a better one by protonation of oxygen to given an oxonium ion. The oxonium ion underg ...
8. Alkynes: An Introduction to Organic Synthesis
... elimination of HX (double dehydrohalogenation) Vicinal dihalides are available from addition of bromine or chlorine to an ...
... elimination of HX (double dehydrohalogenation) Vicinal dihalides are available from addition of bromine or chlorine to an ...
A Guide to Rate of Reactions
... It is important to note that the CAPS document separates Rate of Reaction and Chemical Equilibrium. This is because the underlying theory of each of these is very different. Rate of reaction is also called Chemical Kinetics and deals with how fast a reaction happens. Chemical equilibrium is based on ...
... It is important to note that the CAPS document separates Rate of Reaction and Chemical Equilibrium. This is because the underlying theory of each of these is very different. Rate of reaction is also called Chemical Kinetics and deals with how fast a reaction happens. Chemical equilibrium is based on ...
Aldehydes and Ketones - University of Nebraska Omaha
... • Tollens’ reagent: Prepared by adding NaOH to AgNO3 (aq) to precipitate Ag2O, then adding ammonia to form the silver-ammonia complex ion. • Tollens’ reagent is specific for the oxidation of aldehydes. If silver deposits on the walls of the container as a silver mirror when Tollens’ reagent is mixed ...
... • Tollens’ reagent: Prepared by adding NaOH to AgNO3 (aq) to precipitate Ag2O, then adding ammonia to form the silver-ammonia complex ion. • Tollens’ reagent is specific for the oxidation of aldehydes. If silver deposits on the walls of the container as a silver mirror when Tollens’ reagent is mixed ...
Topics 10 and 20 Outline
... nucleophilic bimolecular substitution reaction. SN1 involves a carbocation intermediate. SN2 involves a concerted reaction with a transition state. • For tertiary halogenoalkanes the predominant mechanism is SN1 and for primary halogenoalkanes it is SN2. Both mechanisms occur for secondary halogenoa ...
... nucleophilic bimolecular substitution reaction. SN1 involves a carbocation intermediate. SN2 involves a concerted reaction with a transition state. • For tertiary halogenoalkanes the predominant mechanism is SN1 and for primary halogenoalkanes it is SN2. Both mechanisms occur for secondary halogenoa ...
Chapter 1 Structure and Bonding
... reversible: H2O, ROH, RSH, RHN2, etc… c) The conditions used in the reaction of these milder bases determine how the reaction proceeds ...
... reversible: H2O, ROH, RSH, RHN2, etc… c) The conditions used in the reaction of these milder bases determine how the reaction proceeds ...
100 Problems and Exercises in Organometallic Chemistry Anil J. Elias
... 17. The molybdenum compound A under UV irradiation liberates two moles of a gas giving a new compound B. The phosphorus NMR spectra of compound A gave a singlet at –17.0 ppm while for B a singlet was observed at +68.2 ppm. The CO stretching bands of both A and B were found to be in the range of 189 ...
... 17. The molybdenum compound A under UV irradiation liberates two moles of a gas giving a new compound B. The phosphorus NMR spectra of compound A gave a singlet at –17.0 ppm while for B a singlet was observed at +68.2 ppm. The CO stretching bands of both A and B were found to be in the range of 189 ...
Accounts
... 3), was reported.27 The bulkiness of BSP occurs on the periphery of the phosphine (at the rim of the bowl) with substantial empty space around the phosphorus atom as shown in Figure 1. On the contrary, P(t-Bu)3 has severe steric congestion within close proximity of the phosphorus atom. Thus, the nat ...
... 3), was reported.27 The bulkiness of BSP occurs on the periphery of the phosphine (at the rim of the bowl) with substantial empty space around the phosphorus atom as shown in Figure 1. On the contrary, P(t-Bu)3 has severe steric congestion within close proximity of the phosphorus atom. Thus, the nat ...
Grignard knowledge: Alkyl coupling chemistry with inexpensive
... nucleophile by the addition of Cu(I) salts enolizable groups are quite incompatible to 3 Molar. As a practical consideration, with the formation of an RMgX functional to form a cuprate species in situ. Certain the reagent concentration is limited by the Grignard reagents such as allylic or group on ...
... nucleophile by the addition of Cu(I) salts enolizable groups are quite incompatible to 3 Molar. As a practical consideration, with the formation of an RMgX functional to form a cuprate species in situ. Certain the reagent concentration is limited by the Grignard reagents such as allylic or group on ...
Chapter 8 Alkenes and Alkynes II
... The π electrons of the double bond are loosely held and are a source of electron density Alkenes are nucleophilic and react with electrophiles such as H+ from a hydrogen halide to form a ...
... The π electrons of the double bond are loosely held and are a source of electron density Alkenes are nucleophilic and react with electrophiles such as H+ from a hydrogen halide to form a ...
CBS Reduction
... • In 1981 Itsuno et al reduced achiral ketones to chiral alcohols using alkoxy-amine-borane complexes in enantioselectivity and in high yield. • In 1987 E.J. Corey, with his co-workers, used oxazaborolidines to rapidlly reduce ketones in the presence of BH3-THF to give 2o alcohol in enantioselectivi ...
... • In 1981 Itsuno et al reduced achiral ketones to chiral alcohols using alkoxy-amine-borane complexes in enantioselectivity and in high yield. • In 1987 E.J. Corey, with his co-workers, used oxazaborolidines to rapidlly reduce ketones in the presence of BH3-THF to give 2o alcohol in enantioselectivi ...
Strong Antiferromagnetic Coupling at Long Distance through a
... is usually predominant in extended π systems, (iii) a ligand to metal electron-transfer that appears for large enough π systems and, (iv) the possible diradical character of long condensed aromatic systems that can be found for n > 5, as shown by Bendikov et al.27 From the results represented in Fig ...
... is usually predominant in extended π systems, (iii) a ligand to metal electron-transfer that appears for large enough π systems and, (iv) the possible diradical character of long condensed aromatic systems that can be found for n > 5, as shown by Bendikov et al.27 From the results represented in Fig ...
Carbon-Carbon Bond Forming Reactions
... - cationic intermediate rearrangements of side chain possible - overalkylation is possible - deactivated benzenes and anilines do not react - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
... - cationic intermediate rearrangements of side chain possible - overalkylation is possible - deactivated benzenes and anilines do not react - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
Here is the Original File - University of New Hampshire
... Recent work has been aimed at understanding the diastereoselectivity of cyclopropane rearrangement of proline-derived systems. In order to probe the selectivity, a rigid tricyclic species was synthesized and NOE analysis was performed. ...
... Recent work has been aimed at understanding the diastereoselectivity of cyclopropane rearrangement of proline-derived systems. In order to probe the selectivity, a rigid tricyclic species was synthesized and NOE analysis was performed. ...
- White Rose Research Online
... predicts that the reaction between palladium acetate and one equivalent of imidazolium 1 is exergonic (Figure 4), with the most stable intermediate being 13. However, DFT calculations indicate that addition of the second equivalent of 1 occurs at 15, as the activation energy is less favourable start ...
... predicts that the reaction between palladium acetate and one equivalent of imidazolium 1 is exergonic (Figure 4), with the most stable intermediate being 13. However, DFT calculations indicate that addition of the second equivalent of 1 occurs at 15, as the activation energy is less favourable start ...
Chapter 12 Alcohols from Carbonyl Compounds: Oxidation
... • Individual blows into a tube containing K2Cr2O7, H2SO4. • The higher the concentration of CH3CH2OH in the breath, the farther the green Cr3+ color extends down the sample tube. • This extent of the green color is then correlated with blood ...
... • Individual blows into a tube containing K2Cr2O7, H2SO4. • The higher the concentration of CH3CH2OH in the breath, the farther the green Cr3+ color extends down the sample tube. • This extent of the green color is then correlated with blood ...
Lecture 14 – Square Planar Complexes
... orbitals (p atomic orbitals, or molecular orbitals of p-symmetry) of ligand L2 (the ligand trans to the leaving group) and Y (the entering group). ...
... orbitals (p atomic orbitals, or molecular orbitals of p-symmetry) of ligand L2 (the ligand trans to the leaving group) and Y (the entering group). ...
Solvent free permanganate oxidations
... Although solvent free reactions are of general interest because of their potential applications in combinatorial chemistry, only a limited number of useful oxidation procedures have been reported.1–12 In this paper we wish to describe a simple and general procedure that can be used for the oxidative ...
... Although solvent free reactions are of general interest because of their potential applications in combinatorial chemistry, only a limited number of useful oxidation procedures have been reported.1–12 In this paper we wish to describe a simple and general procedure that can be used for the oxidative ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.