Beginning Chemistry
... Compounds are substances consisting of two or more elements combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydroge ...
... Compounds are substances consisting of two or more elements combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydroge ...
if Cytochrome substrate and prosthetic-group free radicals
... cells and for which complex and elegant protective mechanisms exist and those which are generated and controlled enzymatically (Boschke 1983 ). Many examples of both types occur in the reductive chemistry of the naturally occurring stable free radical, triplet dioxygen. Scheme 1 outlines some of the ...
... cells and for which complex and elegant protective mechanisms exist and those which are generated and controlled enzymatically (Boschke 1983 ). Many examples of both types occur in the reductive chemistry of the naturally occurring stable free radical, triplet dioxygen. Scheme 1 outlines some of the ...
The Impact of Ligand Design on the Coordination Chemistry and
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
Chapter 4: Aqueous Solutions (Chs 4 and 5 in Jespersen, Ch4 in
... To keep track of the electrons in redox reactions, we assign oxidation numbers (or oxidation states): The oxidation number of an atom in a substance is the actual charge of the atom if it were a monatomic ion. (The oxidation number of an atom is the charge that the atom would have if the compound wa ...
... To keep track of the electrons in redox reactions, we assign oxidation numbers (or oxidation states): The oxidation number of an atom in a substance is the actual charge of the atom if it were a monatomic ion. (The oxidation number of an atom is the charge that the atom would have if the compound wa ...
Chapter One
... It seems logical to start a book of this nature with the question: What is chemistry? Most dictionaries define chemistry as the science that deals with the composition, structure, and properties of substances and the reactions by which one substance is converted into another. Knowing the definition ...
... It seems logical to start a book of this nature with the question: What is chemistry? Most dictionaries define chemistry as the science that deals with the composition, structure, and properties of substances and the reactions by which one substance is converted into another. Knowing the definition ...
GCE Getting Started - Edexcel
... … show electrons being shared (ions are formed by the transfer of electrons!). … remove electrons from the inner shell. … give metals a negative charge. ...
... … show electrons being shared (ions are formed by the transfer of electrons!). … remove electrons from the inner shell. … give metals a negative charge. ...
Stability constants of some rare-earth-metal chelates
... Numerous theoretical studies have been made regarding the way in which stability constants of strong chelates of the EDTA type depend on the nature of the central metal ion and on the structure of the chelating agent (14). Fewer studies have been made involving weak complexes of metal ions with orga ...
... Numerous theoretical studies have been made regarding the way in which stability constants of strong chelates of the EDTA type depend on the nature of the central metal ion and on the structure of the chelating agent (14). Fewer studies have been made involving weak complexes of metal ions with orga ...
View - The Long Group - University of California, Berkeley
... signicant magnetic anisotropy as result of the orbital angular momentum of the t2g5eg2 electron conguration. This behavior extends to complexes with lower coordination numbers, for which the 3d orbital energy splittings are reduced. For example, iron(II) complexes of coordination numbers 4 and 3 h ...
... signicant magnetic anisotropy as result of the orbital angular momentum of the t2g5eg2 electron conguration. This behavior extends to complexes with lower coordination numbers, for which the 3d orbital energy splittings are reduced. For example, iron(II) complexes of coordination numbers 4 and 3 h ...
and Polysaccharides by MALDI-TOF and ESI-ITMS Mass
... the main constituent of starch. The technique was compared with high-performance anionexchange chromatography with pulsed amperometric detection, HPAEC-PAD, an established technique for this purpose. Starch from potato, wheat, and waxy maize was debranched with isoamylase and analyzed using both tec ...
... the main constituent of starch. The technique was compared with high-performance anionexchange chromatography with pulsed amperometric detection, HPAEC-PAD, an established technique for this purpose. Starch from potato, wheat, and waxy maize was debranched with isoamylase and analyzed using both tec ...
Molecular dynamics simulations of valinomycin interactions with
... studying a more complex scenario—for example, protein -ion interactions involving valinomycin together with potassium and sodium ions. It should also be noted that some correlation can be found between the obtained values of the critical electric field strength and the electric potential which is fo ...
... studying a more complex scenario—for example, protein -ion interactions involving valinomycin together with potassium and sodium ions. It should also be noted that some correlation can be found between the obtained values of the critical electric field strength and the electric potential which is fo ...
$doc.title
... Spin-transition compounds exhibit a transition between a diamagnetic low-spin state and a paramagnetic high-spin state. These compounds have potential applications in optical switching, information storage and -display, as well as in temperature and pressure sensors. ...
... Spin-transition compounds exhibit a transition between a diamagnetic low-spin state and a paramagnetic high-spin state. These compounds have potential applications in optical switching, information storage and -display, as well as in temperature and pressure sensors. ...
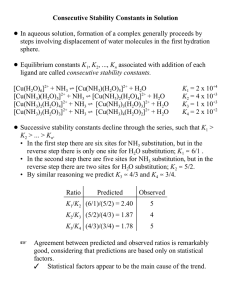
Consecutive Stability Constants in Solution
... • Suggests two paths, where the first term may be pseudo-first-order due to excess solvent acting as an attacking group. ! The ratio of trans and cis isomers is found to vary with the ability of L to act as a trans-directing ligand. ! The increasing order of trans-directing ability is H2O < OH– < py ...
... • Suggests two paths, where the first term may be pseudo-first-order due to excess solvent acting as an attacking group. ! The ratio of trans and cis isomers is found to vary with the ability of L to act as a trans-directing ligand. ! The increasing order of trans-directing ability is H2O < OH– < py ...
QualGroupD
... Ni2+ and Cu2+, the solution is treated to remove Ni2+ and Cu2+ ions, if present, prior to testing for Mg2+ and Zn2+. Ni2+ and Cu2+ Confirmation Tests: Identification of Ni2+: A portion of the unknown solution is made basic with aqueous ammonia and then a drop of dimethylglyoxime ((CH3)2C2(NOH)2, abb ...
... Ni2+ and Cu2+, the solution is treated to remove Ni2+ and Cu2+ ions, if present, prior to testing for Mg2+ and Zn2+. Ni2+ and Cu2+ Confirmation Tests: Identification of Ni2+: A portion of the unknown solution is made basic with aqueous ammonia and then a drop of dimethylglyoxime ((CH3)2C2(NOH)2, abb ...
chemistry - Kachchh University
... 32BeH2, CH4 and BH3. MO diagrams of complex molecules [Co(NH3)6] , [ CoF6] , [Ni(CN6] and ...
... 32BeH2, CH4 and BH3. MO diagrams of complex molecules [Co(NH3)6] , [ CoF6] , [Ni(CN6] and ...
ChemistryPPT
... Videos: https://www.youtube.com/watch?v=hcunQqbNEMQ how plastic made: http://www.youtube.com/watch?v=6eCt0VDg-Kc how recyled bottles made: http://www.youtube.com/watch?v=8QkxpQT967w chem reactions: https://www.youtube.com/watch?v=UkBhW8Kj3r8 https://www.youtube.com/watch?v=BjyXIZtlHFo bill Nye: htt ...
... Videos: https://www.youtube.com/watch?v=hcunQqbNEMQ how plastic made: http://www.youtube.com/watch?v=6eCt0VDg-Kc how recyled bottles made: http://www.youtube.com/watch?v=8QkxpQT967w chem reactions: https://www.youtube.com/watch?v=UkBhW8Kj3r8 https://www.youtube.com/watch?v=BjyXIZtlHFo bill Nye: htt ...
93102 Chemistry Scholarship exemplar-04
... (c) Account for the variation in boiling points of the amines given in the table. 2-methyl – 2-propylamine and 1 – butylamine have the highest boiling points as they both can form hydrogen bonds, ad they have H bonded to the highly electronegative N. 1–butylamine has the higher b.p. of the two as it ...
... (c) Account for the variation in boiling points of the amines given in the table. 2-methyl – 2-propylamine and 1 – butylamine have the highest boiling points as they both can form hydrogen bonds, ad they have H bonded to the highly electronegative N. 1–butylamine has the higher b.p. of the two as it ...
Synthesis and Characterization of Bis-Phosphine
... Transition metal cations have the generic valence shell electronic configuration of ns0(n-1)d0-10 and in general form relatively stable complexes. Transition metal complexes often have one or more unpaired electrons which account for their paramagnetic behavior. In addition, the complexes are usuall ...
... Transition metal cations have the generic valence shell electronic configuration of ns0(n-1)d0-10 and in general form relatively stable complexes. Transition metal complexes often have one or more unpaired electrons which account for their paramagnetic behavior. In addition, the complexes are usuall ...
Course Book - Department of Chemistry
... square pyramidal, trigonal bipyramidal, and tetrahedral complexes; CFSE, pairing energy, low-spin and highspin complexes and magnetic properties; LFT, and molecular orbital (MO) theory of selected octahedral and tetrahedral complexes. Electronic Spectra: UV-Vis, charge transfer, colors, intensities ...
... square pyramidal, trigonal bipyramidal, and tetrahedral complexes; CFSE, pairing energy, low-spin and highspin complexes and magnetic properties; LFT, and molecular orbital (MO) theory of selected octahedral and tetrahedral complexes. Electronic Spectra: UV-Vis, charge transfer, colors, intensities ...
Document
... The Michael addition of active methylene (and methine) compounds to activated π-systems is one of the oldest and more useful carbon–carbon bond-forming reactions (Scheme 1).1,2 However, the required basic catalysis generates by-products arising from competing side reactions. Therefore, catalysis by ...
... The Michael addition of active methylene (and methine) compounds to activated π-systems is one of the oldest and more useful carbon–carbon bond-forming reactions (Scheme 1).1,2 However, the required basic catalysis generates by-products arising from competing side reactions. Therefore, catalysis by ...
Non-native transition metal monoxide nanostructures
... preparation, investigation of their surface structures and physicochemical properties, and useful applications.6 In particular, transition metal monoxides are ideal models for the direct verification of the relationship between antiferromagnetic arrangement of atomic spins and crystal distortions.8 C ...
... preparation, investigation of their surface structures and physicochemical properties, and useful applications.6 In particular, transition metal monoxides are ideal models for the direct verification of the relationship between antiferromagnetic arrangement of atomic spins and crystal distortions.8 C ...
Unit 12: Electrochemistry
... The first step in determining which species was oxidized and which was reduced is knowing what the charge of each species starts at and ends at, so you can determine whether electrons were lost or gained. Why is that important? If you have ever put a battery in backwards in electronics, you’ll und ...
... The first step in determining which species was oxidized and which was reduced is knowing what the charge of each species starts at and ends at, so you can determine whether electrons were lost or gained. Why is that important? If you have ever put a battery in backwards in electronics, you’ll und ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.