Figure 1.01a: (a.)The surface of a single grain of table salt.
... Figure 21.17: (a) The trans isomer of Co(en)2Cl2+ and its mirror image are identical (superimposable). (b) The cis isomer of Co(en)2Cl2+ and its mirror image are not superimposable and are thus a pair of optical isomers. ...
... Figure 21.17: (a) The trans isomer of Co(en)2Cl2+ and its mirror image are identical (superimposable). (b) The cis isomer of Co(en)2Cl2+ and its mirror image are not superimposable and are thus a pair of optical isomers. ...
New. J. Chem., 1992, 16, 633-642
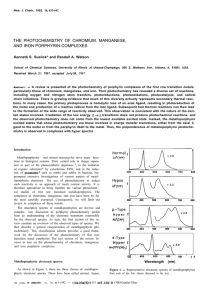
... The photoreductions of both porphyrins and metalloporphyrins are also known. In these cases reducing agents such as ascorbic acid, glutathione, EDTA, or ethyl acetoacetonate are necessary “. In strongly acidic solutions, porphyrins can be rapidly photoreduced to chlorins and bacteriochlorins. These ...
... The photoreductions of both porphyrins and metalloporphyrins are also known. In these cases reducing agents such as ascorbic acid, glutathione, EDTA, or ethyl acetoacetonate are necessary “. In strongly acidic solutions, porphyrins can be rapidly photoreduced to chlorins and bacteriochlorins. These ...
Single molecule synthesis and analysis at the quantum level using
... authors also acknowledge Fred Sachs from the Department of Physiology and Biophysics at SUNY-Buffalo for useful discussions, Matthew Sullivan and Dan Ateya for preparation of the microfabricated templates for the samples at the Cornell Nanofabrication Facility (a member of the National Nanofabricati ...
... authors also acknowledge Fred Sachs from the Department of Physiology and Biophysics at SUNY-Buffalo for useful discussions, Matthew Sullivan and Dan Ateya for preparation of the microfabricated templates for the samples at the Cornell Nanofabrication Facility (a member of the National Nanofabricati ...
Complexometric Reactions and Titrations
... 1. Digestion of the nitrogen containing compound and converting the nitrogen to ammonium hydrogen sulfate. This process is accomplished by decomposing the nitrogen containing compound with sulfuric acid. 2. The solution in step 1 is made alkaline by addition of concentrated NaOH which coverts ammon ...
... 1. Digestion of the nitrogen containing compound and converting the nitrogen to ammonium hydrogen sulfate. This process is accomplished by decomposing the nitrogen containing compound with sulfuric acid. 2. The solution in step 1 is made alkaline by addition of concentrated NaOH which coverts ammon ...
Ion-exchange separation of metals by a single
... complexing agents is usually present to ensure complete coordination of the metals, there is the possibility that one of the metals would form a complex with each of the reagents and thus appear in both the effluent and eluant. ...
... complexing agents is usually present to ensure complete coordination of the metals, there is the possibility that one of the metals would form a complex with each of the reagents and thus appear in both the effluent and eluant. ...
- Kendriya Vidyalaya NKJ Katni
... 9. Why sodium metal is not obtained at cathode when aq NaCl is electrolysed with Pt electrodes but obtained when moleten NaCl is electrolysed ? ...
... 9. Why sodium metal is not obtained at cathode when aq NaCl is electrolysed with Pt electrodes but obtained when moleten NaCl is electrolysed ? ...
Calculating interaction energies in transition metal complexes with
... Comparison of the DF-LMP2/AVTZ data with that of CP corrected MP2 indicates that the local correlation treatment performs well in these cases, producing interaction energies within 0.5 kcal mol−1 of the CP corrected canonical case. This suggests that while the BSSE in the systems is small, the LMP2 ...
... Comparison of the DF-LMP2/AVTZ data with that of CP corrected MP2 indicates that the local correlation treatment performs well in these cases, producing interaction energies within 0.5 kcal mol−1 of the CP corrected canonical case. This suggests that while the BSSE in the systems is small, the LMP2 ...
The Trans Effect. Implications in Enantioselective
... ❑ Groups A and NH3 both occupy part of the 6py and 6pz orbitals. ❑ L is orthogonal to the 6pz orbital, but it overlaps well with the 6py orbital. ❑ Stabilization of the transition state is achieved by increased donation ligand (L) into 6py orbital in ≠. ❑ Calculations for trigonal bipyamidal complex ...
... ❑ Groups A and NH3 both occupy part of the 6py and 6pz orbitals. ❑ L is orthogonal to the 6pz orbital, but it overlaps well with the 6py orbital. ❑ Stabilization of the transition state is achieved by increased donation ligand (L) into 6py orbital in ≠. ❑ Calculations for trigonal bipyamidal complex ...
One-Electron Transformations of Paramagnetic Cobalt
... monomeric and square planar. There is some distortion from planarity in benzyl 5 as the two trans angles, P(1)-Co-P(2) and N-Co-C(31), are 162.93(3)° and 173.6(1)° (180° is ideal). Other structurally characterized compounds39 with Co(II)-C bonds include the cobalt(II) aryl complexes Co(PEt2Ph)2(mesi ...
... monomeric and square planar. There is some distortion from planarity in benzyl 5 as the two trans angles, P(1)-Co-P(2) and N-Co-C(31), are 162.93(3)° and 173.6(1)° (180° is ideal). Other structurally characterized compounds39 with Co(II)-C bonds include the cobalt(II) aryl complexes Co(PEt2Ph)2(mesi ...
Chemistry - Tumkur University
... bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landй equation for calculation of lattice energy, Born-Haber cycle and its applications, polarizing power and polarizability. Fajan’s rules, ionic charac ...
... bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landй equation for calculation of lattice energy, Born-Haber cycle and its applications, polarizing power and polarizability. Fajan’s rules, ionic charac ...
Experimental and Theoretical EPR Study of JahnTeller-
... The EPR spectra of all three complexes and their temperature and solvent dependences are interpreted within a formal ‘2-orbital’ model that reflects the ground-state configuration, and describes the vibronic interactions that lead to the JT distortions. The electronic and vibronic properties of thes ...
... The EPR spectra of all three complexes and their temperature and solvent dependences are interpreted within a formal ‘2-orbital’ model that reflects the ground-state configuration, and describes the vibronic interactions that lead to the JT distortions. The electronic and vibronic properties of thes ...
CHAPTER TWO SOLID STATE REACTIONS 2.0 Introduction The
... phenylacetophenone the solvent-free, mechanically induced, transformation results in the formation of the thermodynamically favorable C-phosphorylated product, which in solution is only obtained together with side products [55]. The mechanical preparation of phosphorus ylides has also been reported ...
... phenylacetophenone the solvent-free, mechanically induced, transformation results in the formation of the thermodynamically favorable C-phosphorylated product, which in solution is only obtained together with side products [55]. The mechanical preparation of phosphorus ylides has also been reported ...
Document
... metalloids with an atomic density greater than 6 g/cm3. Although it is only loosely defined term it is widely recognized and usually applied to the elements such as Cd, Cr, Cu, Hg, Ni, Pb, and Zn which are commonly associated with pollution and toxicity problems. An alternative name for this group o ...
... metalloids with an atomic density greater than 6 g/cm3. Although it is only loosely defined term it is widely recognized and usually applied to the elements such as Cd, Cr, Cu, Hg, Ni, Pb, and Zn which are commonly associated with pollution and toxicity problems. An alternative name for this group o ...
Organic Aqua Regia
... thought by Higashi et al. to be the mechanism for esterification reactions between carboxylic acids and SOCl2 using py as the catalyst (Higashi et al., 1986). However, no systematic spectroscopic study on the SOCl2-py mixture has been reported so far. It is a fact that there is a strong interaction ...
... thought by Higashi et al. to be the mechanism for esterification reactions between carboxylic acids and SOCl2 using py as the catalyst (Higashi et al., 1986). However, no systematic spectroscopic study on the SOCl2-py mixture has been reported so far. It is a fact that there is a strong interaction ...
Photoelectron imaging spectroscopy of Cu (H2O)1,2
... G.J. Rathbone et al. / Chemical Physics Letters 401 (2005) 570–574 ...
... G.J. Rathbone et al. / Chemical Physics Letters 401 (2005) 570–574 ...
Mock Examination (2016/2017) CHEMISTRY PAPER 1 SECTION B
... transition metals. “Zinc and copper are both located in area for transition metals in the Periodic Table. However, zinc is usually not regarded as a transition metals but copper is regarded as a transition metal.” Using Zn and copper reacts with conc H2SO4(l), different colour of the solutions were ...
... transition metals. “Zinc and copper are both located in area for transition metals in the Periodic Table. However, zinc is usually not regarded as a transition metals but copper is regarded as a transition metal.” Using Zn and copper reacts with conc H2SO4(l), different colour of the solutions were ...
The highly preorganized ligands 1,10-Phenanthroline
... attachment to gadolinium, leaving one available coordination site for a water molecule This is of importance because the ability to shift the MRI signal is a function of the number of water molecules directly attached to gadolinium.8 An ideal contrast agent would not have as many points of attachmen ...
... attachment to gadolinium, leaving one available coordination site for a water molecule This is of importance because the ability to shift the MRI signal is a function of the number of water molecules directly attached to gadolinium.8 An ideal contrast agent would not have as many points of attachmen ...
A Well-Dened Terminal Vanadium(III) Oxo Complex
... understanding of their electronic structure and reactivity. However, despite being predicted to be stable by ligand-field theory, the isolation and characterization of a well-defined terminal mononuclear vanadium(III) oxo complex has remained elusive. We present the synthesis and characterization of a ...
... understanding of their electronic structure and reactivity. However, despite being predicted to be stable by ligand-field theory, the isolation and characterization of a well-defined terminal mononuclear vanadium(III) oxo complex has remained elusive. We present the synthesis and characterization of a ...
Chapter 4: Structural features of rhenium carbene complexes
... for the equatorial carbonyl ligands of the two rhenium fragments are the same within experimental error (for [Re2(CO)10] the average eq–CO metal–carbon distance is 1.987 (6) Å). On the {Re(CO)4} fragment, that bears the carbene ligand, there are two carbonyl ligands that are trans to non–carbonyl li ...
... for the equatorial carbonyl ligands of the two rhenium fragments are the same within experimental error (for [Re2(CO)10] the average eq–CO metal–carbon distance is 1.987 (6) Å). On the {Re(CO)4} fragment, that bears the carbene ligand, there are two carbonyl ligands that are trans to non–carbonyl li ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.