5_slides_olefin_complexes_VIPEr
... Created by Margaret L. Scheuermann, Princeton University; Abby R. O’Connor, The College of New Jersey, oconnora@tcnj.edu. Copyright Scheuermann and O’Connor, 2014. This work is licensed under the Creative Commons Attribution-NonCommercialShareAlike 3.0 Unported License. To view a copy of this licens ...
... Created by Margaret L. Scheuermann, Princeton University; Abby R. O’Connor, The College of New Jersey, oconnora@tcnj.edu. Copyright Scheuermann and O’Connor, 2014. This work is licensed under the Creative Commons Attribution-NonCommercialShareAlike 3.0 Unported License. To view a copy of this licens ...
Calculations using Orgel diagrams
... An alternative method is to use Tanabe Sugano diagrams, which are able to predict the transition energies for both spin-allowed and spin-forbidden transitions, as well as for both strong field (low spin), and weak field (high spin) complexes. Note however that most textbooks only give Tanabe-Sugano ...
... An alternative method is to use Tanabe Sugano diagrams, which are able to predict the transition energies for both spin-allowed and spin-forbidden transitions, as well as for both strong field (low spin), and weak field (high spin) complexes. Note however that most textbooks only give Tanabe-Sugano ...
Academic Year 2009/2010 Semester I KTT 212/3 Inorganic
... Know the methods used in the preparation of transition metal complexes. Know about the characterization techniques on transition metal complexes such as FTIR, UV-visible, FTNMR. Understand the presence of different colors associated with various coordination compounds. Understand the basic approach ...
... Know the methods used in the preparation of transition metal complexes. Know about the characterization techniques on transition metal complexes such as FTIR, UV-visible, FTNMR. Understand the presence of different colors associated with various coordination compounds. Understand the basic approach ...
Color and Bonding in Transition Metal Complexes
... Color and Bonding in Transition Metal Complexes ...
... Color and Bonding in Transition Metal Complexes ...
Chapter 13: EDTA titrations
... Chapter 13: EDTA titrations Complexation Reaction: A reaction between two species having a well-defined stoichiometry. The resulting bond is not permanent from a covalent standpoint. Complex: The resulting structure formed during a complexation reaction. Coordination Center: Metal ion in a complex ( ...
... Chapter 13: EDTA titrations Complexation Reaction: A reaction between two species having a well-defined stoichiometry. The resulting bond is not permanent from a covalent standpoint. Complex: The resulting structure formed during a complexation reaction. Coordination Center: Metal ion in a complex ( ...
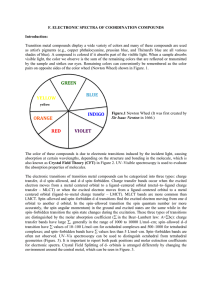
F. ELECTRONIC SPECTRA OF COORDINATION COMPOUNDS
... pairs on opposite sides of the color wheel (Newton Wheel) shown in Figure. 1. ...
... pairs on opposite sides of the color wheel (Newton Wheel) shown in Figure. 1. ...
Transition Metals and Coordination Chemistry
... Which orbitals are considered? 6 orbitals (1 orbital from each of the 6 ligands) come from the ligands. 9 orbitals (1 s orbital, 3 p orbitals, and 5 d orbital) come from the d-metal. Giving a total of 15 molecular orbitals. Where do the electrons come that fill the molecular orbitals? The electrons ...
... Which orbitals are considered? 6 orbitals (1 orbital from each of the 6 ligands) come from the ligands. 9 orbitals (1 s orbital, 3 p orbitals, and 5 d orbital) come from the d-metal. Giving a total of 15 molecular orbitals. Where do the electrons come that fill the molecular orbitals? The electrons ...
Chapter 19: Phenomena
... Which orbitals are considered? 6 orbitals (1 orbital from each of the 6 ligands) come from the ligands. 9 orbitals (1 s orbital, 3 p orbitals, and 5 d orbital) come from the d-metal. Giving a total of 15 molecular orbitals. Where do the electrons come that fill the molecular orbitals? The electrons ...
... Which orbitals are considered? 6 orbitals (1 orbital from each of the 6 ligands) come from the ligands. 9 orbitals (1 s orbital, 3 p orbitals, and 5 d orbital) come from the d-metal. Giving a total of 15 molecular orbitals. Where do the electrons come that fill the molecular orbitals? The electrons ...
Answers to Assignment #5
... field stabilization energy as a multiple of ∆o or ∆T for each of the following complexes using the spectrochemical series to decide, where relevant, which are likely to be strong-field and which weak-field. (a) [Co(NH3 )6 ]3+; (b) [Fe(OH2 )6 ]2+; (c) [Fe(CN)6 ]3– ; (d) [Cr(NH3 )6 ]3+; (e) [W(CO)6 ]; ...
... field stabilization energy as a multiple of ∆o or ∆T for each of the following complexes using the spectrochemical series to decide, where relevant, which are likely to be strong-field and which weak-field. (a) [Co(NH3 )6 ]3+; (b) [Fe(OH2 )6 ]2+; (c) [Fe(CN)6 ]3– ; (d) [Cr(NH3 )6 ]3+; (e) [W(CO)6 ]; ...
No Slide Title - My Illinois State
... Hence, they are called optical isomers. When horizontally polarized light enters an optically active solution. As the light emerges from the solution, the plane of polarity has changed. The mirror image of an enantiomer will rotate the plane of polarized light in the opposite direction. Prentice Hal ...
... Hence, they are called optical isomers. When horizontally polarized light enters an optically active solution. As the light emerges from the solution, the plane of polarity has changed. The mirror image of an enantiomer will rotate the plane of polarized light in the opposite direction. Prentice Hal ...
Handout-5
... The color of coordination complexes arises from electronic transitions between levels whose spacing corresponds to the wavelengths available in the visible light. In complexes, these transitions are frequently referred to as d-d transitions because they involve the orbitals that are mainly d in char ...
... The color of coordination complexes arises from electronic transitions between levels whose spacing corresponds to the wavelengths available in the visible light. In complexes, these transitions are frequently referred to as d-d transitions because they involve the orbitals that are mainly d in char ...
Sample Exercise 24.1 Identifying the Coordination Sphere of a
... has an octahedral geometry. Like [Co(NH3)4Cl2 ]+ (Figure 24.1), it has four ligands of one type and two of another. Consequently, it possesses two isomers: one with the Cl– ligands across the metal from each other (trans-Fe(CO)4Cl2) and one with the Cl– ligands adjacent (cis-Fe(CO)4Cl2). In principl ...
... has an octahedral geometry. Like [Co(NH3)4Cl2 ]+ (Figure 24.1), it has four ligands of one type and two of another. Consequently, it possesses two isomers: one with the Cl– ligands across the metal from each other (trans-Fe(CO)4Cl2) and one with the Cl– ligands adjacent (cis-Fe(CO)4Cl2). In principl ...
IB Chemistry HL Assessment Statements 2009 Revised
... [Ag(NH3)2]+. Only monodentate ligands are required. ...
... [Ag(NH3)2]+. Only monodentate ligands are required. ...
Document
... a metal ion are known as ligands. • Many ligands are known ranging from monoatomic ions such as chloride to huge protein molecules. • Examples include NH3, H2O, NH2CH2CH2NH2 (diaminoethane, a chelating ligand), SCN(thiocyanate) ...
... a metal ion are known as ligands. • Many ligands are known ranging from monoatomic ions such as chloride to huge protein molecules. • Examples include NH3, H2O, NH2CH2CH2NH2 (diaminoethane, a chelating ligand), SCN(thiocyanate) ...
MO Theory
... Assign hybridization of all C atoms (and N) Unused p orbitals maybe available for bonding. Use MO theory to create the bonding for unused p orbitals o double bonds do not allow rotation o triple bonds allow partial rotation o single bonds allow full rotation Arromatic molecules and delocalized ...
... Assign hybridization of all C atoms (and N) Unused p orbitals maybe available for bonding. Use MO theory to create the bonding for unused p orbitals o double bonds do not allow rotation o triple bonds allow partial rotation o single bonds allow full rotation Arromatic molecules and delocalized ...
complex ion
... (ii) Chelating ligands, which do not contain double bonds e.g. ethylenediamine form five membered stable rings. The chelating ligands such as acetylacetone form six membered stable ring complexes. (iii) Ligands with large groups form unstable rings than the ligands with smaller groups due to steric ...
... (ii) Chelating ligands, which do not contain double bonds e.g. ethylenediamine form five membered stable rings. The chelating ligands such as acetylacetone form six membered stable ring complexes. (iii) Ligands with large groups form unstable rings than the ligands with smaller groups due to steric ...
Chapter 27, Nickel, Palladium and Platinum
... produce an axial polarization of the metal and ligand lone pair inducing a positive charge on the near-side of the metal and a negative charge on the far-side which weakens the trans-ligand attachment to the metal. ...
... produce an axial polarization of the metal and ligand lone pair inducing a positive charge on the near-side of the metal and a negative charge on the far-side which weakens the trans-ligand attachment to the metal. ...
Chapter 14 – Inorganic Chemistry
... numbers are 4, 5, and 6, and their common coordination geometries are described below. Octahedral coordination (coordination number = 6) is the most common coordination geometry in this chapter. Coordination Number = 4 ...
... numbers are 4, 5, and 6, and their common coordination geometries are described below. Octahedral coordination (coordination number = 6) is the most common coordination geometry in this chapter. Coordination Number = 4 ...
Electrolytes 1. List whether each of the following is a strong, weak, or
... Coordination compounds in nature Naturally occurring coordination compounds are vital to living organisms. Metal complexes play a variety of important roles in biological systems. Many enzymes, the naturally occurring catalysts that regulate biological processes, are metal complexes (metalloenzymes) ...
... Coordination compounds in nature Naturally occurring coordination compounds are vital to living organisms. Metal complexes play a variety of important roles in biological systems. Many enzymes, the naturally occurring catalysts that regulate biological processes, are metal complexes (metalloenzymes) ...
Slide 1
... Dihydrogen, CH bonds in alkyls groups and alkanes and some other electron-rich -bonds (Si-H, B-H, etc.) can. A simple way to illustrate the ability of H2 to serve as a 2e-donor is given below. H3+ is a know species that forms from H2 and H+: H3+ 2 H + H+ ...
... Dihydrogen, CH bonds in alkyls groups and alkanes and some other electron-rich -bonds (Si-H, B-H, etc.) can. A simple way to illustrate the ability of H2 to serve as a 2e-donor is given below. H3+ is a know species that forms from H2 and H+: H3+ 2 H + H+ ...
Synthesis and Properties of New Oxovanadium (IV) –copper (II
... Cu-Mo antagonism that afflicts ruminants. MoS42- is also clinically used for the removal of excess Cu from Wilson’s disease patients. Only a few reports deal with V/Cu/S complexes.[1] These complexes were obtained from a reaction system containing thiovanadate in the manner used for Mo-S-Cu complexe ...
... Cu-Mo antagonism that afflicts ruminants. MoS42- is also clinically used for the removal of excess Cu from Wilson’s disease patients. Only a few reports deal with V/Cu/S complexes.[1] These complexes were obtained from a reaction system containing thiovanadate in the manner used for Mo-S-Cu complexe ...
Announcements
... – In ionic lattices smaller ion usually fits into octahedral or tetrahedral holes • small (+) ion < 44% radius of big ion into tetrahedral holes • if (+) ion about same size as (-) ion get simple cubic, like CsCl. ...
... – In ionic lattices smaller ion usually fits into octahedral or tetrahedral holes • small (+) ion < 44% radius of big ion into tetrahedral holes • if (+) ion about same size as (-) ion get simple cubic, like CsCl. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.