The Bio-Organometallic Chemistry of Technetium and Rhenium
... given atom. For instance, in the complex [TcCl6]2-, the coordination number of technetium atom is 6 as that is the number of chloride ligands bound to the central technetium atom. This complex is therefore referred to as hexacoordinate. Note that the oxidation state of technetium in this complex is ...
... given atom. For instance, in the complex [TcCl6]2-, the coordination number of technetium atom is 6 as that is the number of chloride ligands bound to the central technetium atom. This complex is therefore referred to as hexacoordinate. Note that the oxidation state of technetium in this complex is ...
9. Coordination Compounds
... 1) Every metal has two types of valencies – primary (10) valency and secondary (20) valency. Primary valency is ionisable, while secondary valency is non-ionisable. 2) Primary valency is denoted by doted lines, while secondary valency is denoted by thick lines. 3) Primary valency gives the oxidation ...
... 1) Every metal has two types of valencies – primary (10) valency and secondary (20) valency. Primary valency is ionisable, while secondary valency is non-ionisable. 2) Primary valency is denoted by doted lines, while secondary valency is denoted by thick lines. 3) Primary valency gives the oxidation ...
Full Text: PDF - Revistas USACH
... were calculated. The proton-ligand formation curves are obtained by plotting nA versus pH at constant temperature. It was found from these that the maximum nA value is 1.7 for HL1 indicating that the ligand has two pKa values. The first dissociation constant, pKa1 (3.94) is assigned to NH proton whi ...
... were calculated. The proton-ligand formation curves are obtained by plotting nA versus pH at constant temperature. It was found from these that the maximum nA value is 1.7 for HL1 indicating that the ligand has two pKa values. The first dissociation constant, pKa1 (3.94) is assigned to NH proton whi ...
View PDF - Oriental Journal of Chemistry
... nitrogen atoms of the Schiff base to the metal ion (N:→M) 29. The (LACu)complex show a broad band at (622 nm) which can be assigned to 2B 1g →2 E g transitions , this band refers to highly distorted octahedral geometry (Jahn-Teller distortion)30. The magnetic moment value is (1.21 B.M) lower than 1. ...
... nitrogen atoms of the Schiff base to the metal ion (N:→M) 29. The (LACu)complex show a broad band at (622 nm) which can be assigned to 2B 1g →2 E g transitions , this band refers to highly distorted octahedral geometry (Jahn-Teller distortion)30. The magnetic moment value is (1.21 B.M) lower than 1. ...
AP Reaction Rules
... For Bronsted-Lowry definition, products are a weak base and weak acid (For Lewis definition, product is a single compound – combination reaction, see complex ion) Complex Ion Formation: a substrate gets surrounded by ligands Substrate is electron-deficient (Lewis acid) – like BF3 or transition metal ...
... For Bronsted-Lowry definition, products are a weak base and weak acid (For Lewis definition, product is a single compound – combination reaction, see complex ion) Complex Ion Formation: a substrate gets surrounded by ligands Substrate is electron-deficient (Lewis acid) – like BF3 or transition metal ...
Coordination Chem
... – Organic bonding theory and simple ideas of ionic charges were not sufficient. • Two types of bonding – Primary – positive charge of the metal ion is balanced by negative ions in the compound. – Secondary – molecules or ion (ligands) are attached directly to the metal ion. • Coordination sphere or ...
... – Organic bonding theory and simple ideas of ionic charges were not sufficient. • Two types of bonding – Primary – positive charge of the metal ion is balanced by negative ions in the compound. – Secondary – molecules or ion (ligands) are attached directly to the metal ion. • Coordination sphere or ...
New Palladium Polymerisation Catalyst Systems
... shows unequalled C-H bond activation in solvated forms of [Cp’(L)Ir(CH,)]+, should organic compounds below room temperature demonstrate even higher reactivity toward and significant selectivity in the C-H bond that organic compounds. ...
... shows unequalled C-H bond activation in solvated forms of [Cp’(L)Ir(CH,)]+, should organic compounds below room temperature demonstrate even higher reactivity toward and significant selectivity in the C-H bond that organic compounds. ...
Chemical Bonding
... • Elements are pure substances that are made of only one type of atom. • Isotopes are elements with different numbers of neutrons. • Because isotopes have the same number electrons, all isotopes of an element have the same chemical properties. ...
... • Elements are pure substances that are made of only one type of atom. • Isotopes are elements with different numbers of neutrons. • Because isotopes have the same number electrons, all isotopes of an element have the same chemical properties. ...
Get Day 17 - Mattson Creighton
... Inorganic Chemistry with Doc M. Day 17. Transition Metals Complexes III: Ligand Field Theory (MO Theory) Topics: 1. Molecular orbital theory and the octahedron 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row trans ...
... Inorganic Chemistry with Doc M. Day 17. Transition Metals Complexes III: Ligand Field Theory (MO Theory) Topics: 1. Molecular orbital theory and the octahedron 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row trans ...
Topic 6 Coordination Compounds Coordination Chemistry
... Charge on the metal (oxidation state) For first row transition elements DO varies from about 7,500 cm–1 to 12,500 cm–1 for divalent ions, and 14,000 cm–1 to 25,000 cm –1 for trivalent ions. Position in a group values for analogous complexes of metal ions in a group increases by 25% to 50% going from ...
... Charge on the metal (oxidation state) For first row transition elements DO varies from about 7,500 cm–1 to 12,500 cm–1 for divalent ions, and 14,000 cm–1 to 25,000 cm –1 for trivalent ions. Position in a group values for analogous complexes of metal ions in a group increases by 25% to 50% going from ...
Organometallic Chemistry
... Such cycloaddition reactions between two alkenes to give cyclobutanes are symmetry forbidden and occur only photochemically. However, the presence of dorbitals on the metal alkylidene fragment breaks this symmetry and the reaction is quite facile. Normally, the products are statistical, unless the r ...
... Such cycloaddition reactions between two alkenes to give cyclobutanes are symmetry forbidden and occur only photochemically. However, the presence of dorbitals on the metal alkylidene fragment breaks this symmetry and the reaction is quite facile. Normally, the products are statistical, unless the r ...
chemistry 112 worksheet
... The coordinate complex itself consists of a transition metal atom or ion and the surrounding ligands. The coordinate complex is always enclosed in square brackets, [ ]. The coordinate complex can be an ion, cation or anion, or a neutral complex. Counter ions are needed to produce a neutral coordinat ...
... The coordinate complex itself consists of a transition metal atom or ion and the surrounding ligands. The coordinate complex is always enclosed in square brackets, [ ]. The coordinate complex can be an ion, cation or anion, or a neutral complex. Counter ions are needed to produce a neutral coordinat ...
for quiz on 13 Mar
... the entire ion to have a +2 charge, Co will need to have a +3 charge. Thus, the oxidation state of Co is +3. 20.75 a. The charge on an ammonium ion (NH4+) is +1. The charge on a Cl- is -1. There are 6 Cl-, contributing -6 total, and there are 3 ammoniums, contributing +3. To make an overall neutral ...
... the entire ion to have a +2 charge, Co will need to have a +3 charge. Thus, the oxidation state of Co is +3. 20.75 a. The charge on an ammonium ion (NH4+) is +1. The charge on a Cl- is -1. There are 6 Cl-, contributing -6 total, and there are 3 ammoniums, contributing +3. To make an overall neutral ...
Crowns and Crypts
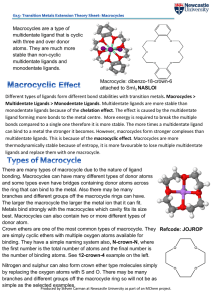
... Crown ethers (or crowns) are a group of macrocyclic polyethers in which the ethereal 0 atoms are separated by two methy·lene (CH 2-) groups. A typical example of a crown ether is 2, 3, 11, 12dibenzo 1, 4, 7, 10, 13, 16-hexaoxacyclooctadeca-2, single 11diene designated as dibenzo-18-Crown-6 (or simpl ...
... Crown ethers (or crowns) are a group of macrocyclic polyethers in which the ethereal 0 atoms are separated by two methy·lene (CH 2-) groups. A typical example of a crown ether is 2, 3, 11, 12dibenzo 1, 4, 7, 10, 13, 16-hexaoxacyclooctadeca-2, single 11diene designated as dibenzo-18-Crown-6 (or simpl ...
The Transition Metals
... electron. Starting with Sc and moving left‐to‐right across the periodic table, each move to the right leads to a +1 increase in the nuclear charge and the addition of 1 electron to the 3d subshell. Because they are in a lower n shell, the 3d electrons are good at shielding the 4s electrons. That m ...
... electron. Starting with Sc and moving left‐to‐right across the periodic table, each move to the right leads to a +1 increase in the nuclear charge and the addition of 1 electron to the 3d subshell. Because they are in a lower n shell, the 3d electrons are good at shielding the 4s electrons. That m ...
Chapter 2: Dative Ligands 2.1 Introduction 2.2.1. Properties of Free
... (relative stability depends on the number and identity of ligand) electrophilic at carbene carbon (C ) nucleophilic at metal and C ...
... (relative stability depends on the number and identity of ligand) electrophilic at carbene carbon (C ) nucleophilic at metal and C ...
5. Bonding in Complexes
... Optical isomerism • If the lifetimes of the two enantiomers of a chiral molecule are long enough to be separable they are called optical isomers. ...
... Optical isomerism • If the lifetimes of the two enantiomers of a chiral molecule are long enough to be separable they are called optical isomers. ...
Ch. 15 Sections 15.6-15.8 Powerpoint
... •General rule – If the anion X- is an effective base (HX is a weak acid) the salt MX will show increased solubility in an acidic solution. ...
... •General rule – If the anion X- is an effective base (HX is a weak acid) the salt MX will show increased solubility in an acidic solution. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.