SYNTHESIS AND ANTIMICROBIAL STUDIES OF IRON (III
... the metal. These d-orbitals, according to ligand field theory, split into high and low energy groups under the influence of the asymmetric (typically octahedral) electric field created by the array of ligands. This accounts for the highly coloured nature of the complexes when (as frequently happens) ...
... the metal. These d-orbitals, according to ligand field theory, split into high and low energy groups under the influence of the asymmetric (typically octahedral) electric field created by the array of ligands. This accounts for the highly coloured nature of the complexes when (as frequently happens) ...
Studies on nickel(II) and palladium(II) complexes with some
... The electronic spectral data of all the six Ni(II) complexes exhibited three spin allowed transitions from 3A2g → 3T2g, 3T1g(F), and 3T1g(P), which appeared 9850–10700 cm–1, 13600–15052 cm–1 and 24096–28668 cm–1, respectively. This spectral pattern corresponds to an octahedral/distorted octahedral g ...
... The electronic spectral data of all the six Ni(II) complexes exhibited three spin allowed transitions from 3A2g → 3T2g, 3T1g(F), and 3T1g(P), which appeared 9850–10700 cm–1, 13600–15052 cm–1 and 24096–28668 cm–1, respectively. This spectral pattern corresponds to an octahedral/distorted octahedral g ...
Study Guide Answers
... 14. All substances are either atoms, elements, molecules, or compounds. 15. Is air matter? Explain your answer. Air is matter because it has mass and takes up space. 16. Explain why atoms in their natural state are neutral. Atoms in their natural state are neutral because they have the same number ...
... 14. All substances are either atoms, elements, molecules, or compounds. 15. Is air matter? Explain your answer. Air is matter because it has mass and takes up space. 16. Explain why atoms in their natural state are neutral. Atoms in their natural state are neutral because they have the same number ...
Applications of N-Heterocyclic Carbenes in Organic Reactions
... NHC ligands is the recent advance. Ø Although NHCs can be considered as phosphane mimics it has become apparent that there are substantial differences between the two ligand families. Ø Many of these carbene species show high catalytic activity in many metal-mediated organic transformations, e.g. ru ...
... NHC ligands is the recent advance. Ø Although NHCs can be considered as phosphane mimics it has become apparent that there are substantial differences between the two ligand families. Ø Many of these carbene species show high catalytic activity in many metal-mediated organic transformations, e.g. ru ...
Reactivity of Transition Metal Organometallics L. J. Farrugia MSc
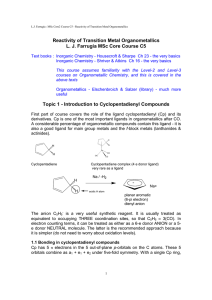
... The anion C5H5- is a very useful synthetic reagent. It is usually treated as equivalent to occupying THREE coordination sites, so that C5H5 ≡ 3(CO). In electron counting terms, it can be treated as either as a 6-e donor ANION or a 5e donor NEUTRAL molecule. The latter is the recommended approach bec ...
... The anion C5H5- is a very useful synthetic reagent. It is usually treated as equivalent to occupying THREE coordination sites, so that C5H5 ≡ 3(CO). In electron counting terms, it can be treated as either as a 6-e donor ANION or a 5e donor NEUTRAL molecule. The latter is the recommended approach bec ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
... 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involve the uniting or the separation of atoms of different elements Dalton ...
(EAN) Rule
... Little or no pi interaction between metals and ligands. Energy of the t2g orbitals is the same as the free metal. Their occupation has no impact on the stability of the complex. There are 6 low energy bonding MO’s, 3 medium energy MO’s and and 6 strongly antibonding MO’s (too high energy to be occup ...
... Little or no pi interaction between metals and ligands. Energy of the t2g orbitals is the same as the free metal. Their occupation has no impact on the stability of the complex. There are 6 low energy bonding MO’s, 3 medium energy MO’s and and 6 strongly antibonding MO’s (too high energy to be occup ...
compound - Coal City Unit #1
... • show the exact number of atoms present in a molecule and how these atoms are bonded to one another • lines represent the bonds betw. atoms ...
... • show the exact number of atoms present in a molecule and how these atoms are bonded to one another • lines represent the bonds betw. atoms ...
Crystal Field Splitting in Octahedral Transition Metal Complexes
... To a first approximation, the ligand field is of Oh symmetry, and the 3d orbitals will separate into a set of three degenerate orbitals (t2g = dxy, dyz, dxz) and a set of two degenerate orbitals (eg = dx2–y2, dz2). ...
... To a first approximation, the ligand field is of Oh symmetry, and the 3d orbitals will separate into a set of three degenerate orbitals (t2g = dxy, dyz, dxz) and a set of two degenerate orbitals (eg = dx2–y2, dz2). ...
Chapter 3: Molecules, Compounds and Chemical Equations
... Ionic compounds can be chemically attached to a small number of water molecules in a solid form. CuSO4.5H2O, copper (II) sulfate pentahydrate ...
... Ionic compounds can be chemically attached to a small number of water molecules in a solid form. CuSO4.5H2O, copper (II) sulfate pentahydrate ...
Type of Bonding
... • charge-nonpolar (induced or instantaneous dipole) • dipole-nonpolar (induced dipole) ...
... • charge-nonpolar (induced or instantaneous dipole) • dipole-nonpolar (induced dipole) ...
Slide 1
... 17C Organic complexing agents These have inherent sensitivity and potential selectivity in reacting with metal ions. Organic reagents are particularly useful in precipitating metals, in binding metals so as to prevent interferences, in extracting metals from one solvent to another, and in forming c ...
... 17C Organic complexing agents These have inherent sensitivity and potential selectivity in reacting with metal ions. Organic reagents are particularly useful in precipitating metals, in binding metals so as to prevent interferences, in extracting metals from one solvent to another, and in forming c ...
and Square-Grooved Networks to Tubular Assemblies in
... contains a unique uranyl ion located on a mirror plane and chelated by three carboxylate groups from three tca3– ligands (Fig. 1). The U–O(oxo) bond lengths of 1.757(8) and 1.758(7) Å, and the U–O(carboxylate) bond lengths of 2.462(5)–2.468(5) Å are unexceptional. Both metal and ligand are threefold ...
... contains a unique uranyl ion located on a mirror plane and chelated by three carboxylate groups from three tca3– ligands (Fig. 1). The U–O(oxo) bond lengths of 1.757(8) and 1.758(7) Å, and the U–O(carboxylate) bond lengths of 2.462(5)–2.468(5) Å are unexceptional. Both metal and ligand are threefold ...
2011 Chem Facts Key
... 33. Ionic bonds form when one atom transfers an electron to another atom when forming a bond with it. Which substance exhibits ionic bonding rather than covalent bonding? CO2 , N2O4, SiO2 , CaBr2 , C6H12O6 34. Lewis Dot Diagrams may be used to represent the formation of polyatomic ions or covalent m ...
... 33. Ionic bonds form when one atom transfers an electron to another atom when forming a bond with it. Which substance exhibits ionic bonding rather than covalent bonding? CO2 , N2O4, SiO2 , CaBr2 , C6H12O6 34. Lewis Dot Diagrams may be used to represent the formation of polyatomic ions or covalent m ...
File
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
... electrons through chemical reactions. – This gives them an outer shell configuration like their nearest noble gas and therefore they become stable. – From the family number of the representative elements, you can determine the number of valence electrons, and therefore the number of electrons necess ...
Chapter 4 The Structure of Matter
... or ions within a substance. • a. Two terms are used to specify the relative positions of atoms to each other in a compound. − (1) Bond length - gives the distance between the two nuclei of the atoms − (2) Bond angles tell how these atoms are oriented when you have three or more atoms in the compound ...
... or ions within a substance. • a. Two terms are used to specify the relative positions of atoms to each other in a compound. − (1) Bond length - gives the distance between the two nuclei of the atoms − (2) Bond angles tell how these atoms are oriented when you have three or more atoms in the compound ...
2016 Pre Course CHEMISTRY - Calday Grange Grammar School
... Deduce the type of bonding present in Na2S and that present in CS2 Bonding in Na2S ........................................................................................ Bonding in CS2........................................................................................... ...
... Deduce the type of bonding present in Na2S and that present in CS2 Bonding in Na2S ........................................................................................ Bonding in CS2........................................................................................... ...
Rare earth complexes in sol-gel glasses R *
... 3. Complexes of rare earths in sol-gel glasses As described above, electronic transitions in trivalent rare earth ions within the f shell are forbidden by the Laporte rule and the luminescence which is dependent on the absorption of photons to the electronic levels of the same configuration is there ...
... 3. Complexes of rare earths in sol-gel glasses As described above, electronic transitions in trivalent rare earth ions within the f shell are forbidden by the Laporte rule and the luminescence which is dependent on the absorption of photons to the electronic levels of the same configuration is there ...
The elements of the first transition series
... The metal or high Ni alloys are used to handle F2 and other corrosive fluorides. Nickel absorbs considerable amounts of hydrogen when finely divided and special forms ofNi (e.g., Raney nickel) are used for catalytic reductions. The Chemistry of Nickel(II), d8 The binary compounds, such as NiO ...
... The metal or high Ni alloys are used to handle F2 and other corrosive fluorides. Nickel absorbs considerable amounts of hydrogen when finely divided and special forms ofNi (e.g., Raney nickel) are used for catalytic reductions. The Chemistry of Nickel(II), d8 The binary compounds, such as NiO ...
Properties of Metals vs. Nonmetals vs. Metalloids
... Atomic Masses: What is the difference between the mass number for Carbon–14 and carbon’s atomic mass of 12.011 amu? ...
... Atomic Masses: What is the difference between the mass number for Carbon–14 and carbon’s atomic mass of 12.011 amu? ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.