Common Structural Features in Calcium
... diffractometer. XRPD patterns corresponding to the single phases were autoindexed using the DICVOL06 program,18 and the space groups were derived from the observed systematic extinctions. To minimize the preferred orientation effects, XRPD patterns of 2 and 4 for ab initio structure determination (s ...
... diffractometer. XRPD patterns corresponding to the single phases were autoindexed using the DICVOL06 program,18 and the space groups were derived from the observed systematic extinctions. To minimize the preferred orientation effects, XRPD patterns of 2 and 4 for ab initio structure determination (s ...
π bonded ligands (# 2)
... 1g molecular orbital arises from the bonding combination of the ligand e1 orbitals with the Fe 3dxz and 3dyz orbitals. This is the only symmetry combination of orbitals in the two Cp rings that has appreciable overlap with the metal 3d orbitals to act as an efficient donor and it is thus this intera ...
... 1g molecular orbital arises from the bonding combination of the ligand e1 orbitals with the Fe 3dxz and 3dyz orbitals. This is the only symmetry combination of orbitals in the two Cp rings that has appreciable overlap with the metal 3d orbitals to act as an efficient donor and it is thus this intera ...
Section 8.3 Names and Formulas of Ionic Compounds Formula Unit
... 2. Monoatomic cations use the element. 3. Monoatomic anions take their name from the root of the element name plus the suffix -ide. 4. Group 1A and 2A metals have only one oxidation number. Transition metals and metals on the right side of the periodic table often have more that one oxidation number ...
... 2. Monoatomic cations use the element. 3. Monoatomic anions take their name from the root of the element name plus the suffix -ide. 4. Group 1A and 2A metals have only one oxidation number. Transition metals and metals on the right side of the periodic table often have more that one oxidation number ...
Chapter 2 - Chemical Context of Life
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
Unit 1 - Measurement Atomic Theory
... (a) Combination of usually one or more non-metal atoms (b) Held together by covalent bonding (c) Intramolecular forces are strong; intermolecular forces are weak (d) Structural formulas ...
... (a) Combination of usually one or more non-metal atoms (b) Held together by covalent bonding (c) Intramolecular forces are strong; intermolecular forces are weak (d) Structural formulas ...
Atoms, Molecules and Ions
... (c) Each calcium ion (Ca2+) bears two positive charges, and each phosphate ion (PO4-3) bears three negative charges. ...
... (c) Each calcium ion (Ca2+) bears two positive charges, and each phosphate ion (PO4-3) bears three negative charges. ...
19 - WSU chemistry
... (2.476(10) Å) is significantly longer than the other two bonds to C(2) (Ir(1)−C(2) = 1.917(9) Å and Ru(2)−C(2) = 2.150(10) Å) but seems to contain important bonding interactions (see below). The oxygen atom is bonded to Ru(1) (Ru(1)−O(2) = 2.168(8) Å), and as a result, the CO bond distance is long (C ...
... (2.476(10) Å) is significantly longer than the other two bonds to C(2) (Ir(1)−C(2) = 1.917(9) Å and Ru(2)−C(2) = 2.150(10) Å) but seems to contain important bonding interactions (see below). The oxygen atom is bonded to Ru(1) (Ru(1)−O(2) = 2.168(8) Å), and as a result, the CO bond distance is long (C ...
Chem 174–Lecture 9b_..
... In extreme cases, both bonds can be characterized as double bonds (II). In cases in which the M-N-O angle is close to 180o, the M-N bond is usually relatively short. If the backbonding effect is weak, the angle decreases significantly (< (M-N-O)= ~120 o) and the ligand can be described as “NO-“ ...
... In extreme cases, both bonds can be characterized as double bonds (II). In cases in which the M-N-O angle is close to 180o, the M-N bond is usually relatively short. If the backbonding effect is weak, the angle decreases significantly (< (M-N-O)= ~120 o) and the ligand can be described as “NO-“ ...
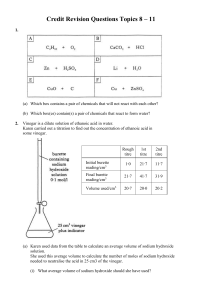
Credit Revision Questions Topics 8 – 11 1. (a) Which box contains a
... (b) (i) What term is used to describe the type of chemical reaction taking place in beaker B? (ii) Suggest what would happen to the pH in beaker B. (c) Write the ion-electron equation for the chemical reaction taking place in beaker A. 10. Some Euro coins are made from a hard-wearing alloy called No ...
... (b) (i) What term is used to describe the type of chemical reaction taking place in beaker B? (ii) Suggest what would happen to the pH in beaker B. (c) Write the ion-electron equation for the chemical reaction taking place in beaker A. 10. Some Euro coins are made from a hard-wearing alloy called No ...
Synthesis, characterization and antimicrobial activities of schiff base
... the region 334-398 nm which is assigned to the charge transfer transition (26). A magnetic moment of 1.91 BM which is higher than the spin only value (1.73) BM expected for one unpaired electron confirmed the octahedral geometry of the complex (27). Ni(II) complexes are diamagnetic and show bands in ...
... the region 334-398 nm which is assigned to the charge transfer transition (26). A magnetic moment of 1.91 BM which is higher than the spin only value (1.73) BM expected for one unpaired electron confirmed the octahedral geometry of the complex (27). Ni(II) complexes are diamagnetic and show bands in ...
xes, except for the mono-complex which was regu
... xes, except for the mono-complex which was regular octahedral, had shorter Ni-O bonds than Ni-N bonds, and the differences between the Ni-O and Ni-N distances were more enhanced in the tris-complex than in the bis-complex. Stepwise formation constants of the glycinato complexes decreased with the nu ...
... xes, except for the mono-complex which was regular octahedral, had shorter Ni-O bonds than Ni-N bonds, and the differences between the Ni-O and Ni-N distances were more enhanced in the tris-complex than in the bis-complex. Stepwise formation constants of the glycinato complexes decreased with the nu ...
Abstract - Trade Science Inc
... the complex equals 10923 cm-1, and B value found to be 733.1 cm-1, while β equals 0.65 (Table 3). These assignments correspond to Ni(II) octahedral complexes16,18,20,21. ...
... the complex equals 10923 cm-1, and B value found to be 733.1 cm-1, while β equals 0.65 (Table 3). These assignments correspond to Ni(II) octahedral complexes16,18,20,21. ...
Conformation Switching in Gas-Phase Complexes of Histidine with
... availability of Lewis-base sites and basicity of the side chain; and steric strain in chelation between the amino acid and the metal ion. The present work focuses on the variation of these factors along the series of doubly charged alkaline earth metal ions. Histidine is an interesting chelating lig ...
... availability of Lewis-base sites and basicity of the side chain; and steric strain in chelation between the amino acid and the metal ion. The present work focuses on the variation of these factors along the series of doubly charged alkaline earth metal ions. Histidine is an interesting chelating lig ...
Stability of Coordination Compounds
... elements is also related to the absence of any eg electrons. In the crystal field model the presence of eg electrons means that there are increased electronelectron repulsions with ligand electron pairs and therefore longer and weaker bonds. In the molecular orbital model the eg orbitals are antibon ...
... elements is also related to the absence of any eg electrons. In the crystal field model the presence of eg electrons means that there are increased electronelectron repulsions with ligand electron pairs and therefore longer and weaker bonds. In the molecular orbital model the eg orbitals are antibon ...
Metal ions in non-complementary DNA base pairs: an ab initio study
... neglect of intermolecular electron correlation effects, which is also observed in our complex. However, it does not have a significant effect on the calculated energetics of the complexes, since for the interaction energy single point calculations the electron correlation is included (see below). Fu ...
... neglect of intermolecular electron correlation effects, which is also observed in our complex. However, it does not have a significant effect on the calculated energetics of the complexes, since for the interaction energy single point calculations the electron correlation is included (see below). Fu ...
Lecture 21
... Recall that the set of d atomic electrons are split into a t2g set and an eg set by repulsion with the negative octahedral ligands, similarly for tetrahedral environment. So also are the free ion terms split by their environment. dn ...
... Recall that the set of d atomic electrons are split into a t2g set and an eg set by repulsion with the negative octahedral ligands, similarly for tetrahedral environment. So also are the free ion terms split by their environment. dn ...
msc_pre_chemistry_pap1_bl3
... organic ligand itself. That resonance may affect the formation of a chelate was first shown by Calvin and Wilson. The double bond resonance has been attributed as a reason to be unusual stability of histamine cobalt chelate. Orbital hybridisation There are certain factors which serves to make a spec ...
... organic ligand itself. That resonance may affect the formation of a chelate was first shown by Calvin and Wilson. The double bond resonance has been attributed as a reason to be unusual stability of histamine cobalt chelate. Orbital hybridisation There are certain factors which serves to make a spec ...
Copper(II) Mixed Ligands Complexes of Hydroxamic Acids with
... This work reports the results of a combined potentiometric and spectrophotometric study of CuII mixed ligand complexes formed by one hydroxamic acid (Aha and Gha) taken as the primary ligand (A) and a secondary ligand (B) represented either by histamine (Ha) or by the aminoacids glycine (Gly) and hi ...
... This work reports the results of a combined potentiometric and spectrophotometric study of CuII mixed ligand complexes formed by one hydroxamic acid (Aha and Gha) taken as the primary ligand (A) and a secondary ligand (B) represented either by histamine (Ha) or by the aminoacids glycine (Gly) and hi ...
COMPLEX IONS AND AMPHOTERISM
... An amphoteric substance is one that can behave as a Lewis acid and a Brønsted base. The best examples are found with metal hydroxides such as aluminum hydroxide [Al(OH)3] and zinc hydroxide [Zn(OH)2]. Insoluble aluminum hydroxide can be formed by the addition of hydroxide ion, OH-, to a soluble salt ...
... An amphoteric substance is one that can behave as a Lewis acid and a Brønsted base. The best examples are found with metal hydroxides such as aluminum hydroxide [Al(OH)3] and zinc hydroxide [Zn(OH)2]. Insoluble aluminum hydroxide can be formed by the addition of hydroxide ion, OH-, to a soluble salt ...
Chemistry of Art by Jimmy Huang
... wavelengths related to their atomic structures. When a substance absorbs certain wavelengths of light in the visible region, the colour of the substance is determined by the wavelengths of visible light that remain. The substance exhibits the colour complementary to those absorbed. Bohr Model of the ...
... wavelengths related to their atomic structures. When a substance absorbs certain wavelengths of light in the visible region, the colour of the substance is determined by the wavelengths of visible light that remain. The substance exhibits the colour complementary to those absorbed. Bohr Model of the ...
Sequence and Structural Analysis of Ligand Binding Sites in
... structures, excluding the NMR structures, fragments and repeated structures, 173 are found to be complexed with various ligands. Based on the bound ligands they are further classified into different groups (Table 1). These groups contain both α-helical and β-barrel proteins as well. Atom contacts an ...
... structures, excluding the NMR structures, fragments and repeated structures, 173 are found to be complexed with various ligands. Based on the bound ligands they are further classified into different groups (Table 1). These groups contain both α-helical and β-barrel proteins as well. Atom contacts an ...
CERAMICS MATERIALS - Wits Structural Chemistry
... The 3d-metal oxides such as MnO, FeO, CoO and NiO are semiconductors and TiO and VO are metallic conductors. 3d-metal oxides - MnO, Fe1-xO, CoO and NiO have low conductivity that increase with temperature or have such large band gaps that become insulators. The electron-hole migration in these oxide ...
... The 3d-metal oxides such as MnO, FeO, CoO and NiO are semiconductors and TiO and VO are metallic conductors. 3d-metal oxides - MnO, Fe1-xO, CoO and NiO have low conductivity that increase with temperature or have such large band gaps that become insulators. The electron-hole migration in these oxide ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS 1 CHAPTER TWO
... a. The smaller parts are electrons and the nucleus. The nucleus is broken down into protons and neutrons which can be broken down into quarks. For our purpose, electrons, neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isot ...
... a. The smaller parts are electrons and the nucleus. The nucleus is broken down into protons and neutrons which can be broken down into quarks. For our purpose, electrons, neutrons, and protons are the key smaller parts of an atom. b. All atoms of hydrogen have 1 proton in the nucleus. Different isot ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.