Chm_451_F-12_Exam_3_..
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
CHAPTER V Cu(II), Ni(II) and Co(II) Schiff bases complexes derived
... physiological conditions via oxidative and hydrolytic mechanisms are of important. Binding studies of transition metal complexes have become a very important field in the development of DNA molecule probes and chemotherapeutics in recent years [4-11]. In order to find anticarcinogens that can recogn ...
... physiological conditions via oxidative and hydrolytic mechanisms are of important. Binding studies of transition metal complexes have become a very important field in the development of DNA molecule probes and chemotherapeutics in recent years [4-11]. In order to find anticarcinogens that can recogn ...
a contribution to weaken health problems in bolivia: bioactive
... addition to its extraordinary cumulative properties, cadmium is also a highly toxic metal that can disrupt a number of biological systems, usually doses that are much lower than most toxic metals [2]. Cadmium has several adverse effect in the biological systems [3-10]. Iron is vital for almost all l ...
... addition to its extraordinary cumulative properties, cadmium is also a highly toxic metal that can disrupt a number of biological systems, usually doses that are much lower than most toxic metals [2]. Cadmium has several adverse effect in the biological systems [3-10]. Iron is vital for almost all l ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 1. Write the correct electronic configuration of Cr and Cu. 2. What are variable oxidation states? Give an example. 3. Name the element whose atomic number is 100. Write its electronic configuration. 4. What is the chief source of uranium? 5. Define the term chelate effect. 6. Write the IUPAC name o ...
... 1. Write the correct electronic configuration of Cr and Cu. 2. What are variable oxidation states? Give an example. 3. Name the element whose atomic number is 100. Write its electronic configuration. 4. What is the chief source of uranium? 5. Define the term chelate effect. 6. Write the IUPAC name o ...
Antimicrobial activity of some of chlorocobaloximes containing
... The UV spectra of the cobaloximes showed a shoulder around 330 nm which may be due to the ligand to metal charge transfer (LMCT) [24]. The electronic spectra of the complexes showed an high intense absorption band in the range 230-270 nm which may be attributed to π→π* transition of the pyridine rin ...
... The UV spectra of the cobaloximes showed a shoulder around 330 nm which may be due to the ligand to metal charge transfer (LMCT) [24]. The electronic spectra of the complexes showed an high intense absorption band in the range 230-270 nm which may be attributed to π→π* transition of the pyridine rin ...
OXOVANADIUM(IV) COMPLEXES WITH LIGANDS DERIVED BY
... Vijai SINGH, Probin BORA and Hardeo Singh YADAV* Department of Chemistry, North Eastern Regional Institute of Science and Technology, ...
... Vijai SINGH, Probin BORA and Hardeo Singh YADAV* Department of Chemistry, North Eastern Regional Institute of Science and Technology, ...
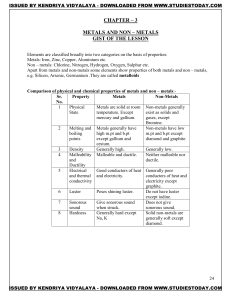
METALS AND NON – METALS Concepts
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
Periodic Table - Mendeleev (1869): --
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
File
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
... 6. The oxidation state of hydrogen in most of its compounds is +1 unless it combines with a metal, in which cases it is -1. 7. In compounds, the elements of groups 1 and 2 as well as aluminum have oxidation numbers of +1, +2, and +3, respectively. 8. The sum of the oxidation numbers of all atoms in ...
Voltammetric Determination of Trace Concentrations of Metals in the
... (28,29) (obtained using bioassays). The ASV method can also be combined with other separation methods to subdivide the labile and inert groups (it should be re-emphasized that the results of these experiments will depend on the nature of the separation method; that is, they are operationally defined ...
... (28,29) (obtained using bioassays). The ASV method can also be combined with other separation methods to subdivide the labile and inert groups (it should be re-emphasized that the results of these experiments will depend on the nature of the separation method; that is, they are operationally defined ...
video slide
... a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom in a different molecule In living cells, the electronegative partners are usually oxygen or nitrogen atoms ...
... a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom in a different molecule In living cells, the electronegative partners are usually oxygen or nitrogen atoms ...
Review for Exam 1
... Determine how many of each ion type is needed for an overall charge of zero. When the cation and anion have different charges, use the ion charges to determine the number of ions of each needed. ...
... Determine how many of each ion type is needed for an overall charge of zero. When the cation and anion have different charges, use the ion charges to determine the number of ions of each needed. ...
AP Chemistry Summer Assignment - 2015
... EX. 2NaCl(l) → 2Na(s) + Cl2(g) : Use the solubility rules to decide whether a product of an ionic reaction is insoluble in water and will thus form a precipitate. If a compound is soluble in water then it should be shown as being in aqueous solution, or left as separate ions. It is, in fact, often m ...
... EX. 2NaCl(l) → 2Na(s) + Cl2(g) : Use the solubility rules to decide whether a product of an ionic reaction is insoluble in water and will thus form a precipitate. If a compound is soluble in water then it should be shown as being in aqueous solution, or left as separate ions. It is, in fact, often m ...
Magnetic properties of complexes
... ‘E’ state is doubly degenerate, which means that there are two energy levels with the same energy. The orbitals giving rise to this are dx2-y2 and dz2. One orbital cannot be changed into another by rotation because their shapes are different. Hence, A and E terms do not have orbital angular momentum ...
... ‘E’ state is doubly degenerate, which means that there are two energy levels with the same energy. The orbitals giving rise to this are dx2-y2 and dz2. One orbital cannot be changed into another by rotation because their shapes are different. Hence, A and E terms do not have orbital angular momentum ...
Inorganic Exam 3 Name: Chm 451 2 December 2010
... (c) the atomic radius is smaller than predicted and the first ionization energy is larger than predicted (d) the atomic radius is larger than predicted and the first ionization energy is smaller than predicted 5. (6 pts) Sketch the structure of these transition metal compounds (ignore the counter-io ...
... (c) the atomic radius is smaller than predicted and the first ionization energy is larger than predicted (d) the atomic radius is larger than predicted and the first ionization energy is smaller than predicted 5. (6 pts) Sketch the structure of these transition metal compounds (ignore the counter-io ...
NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS OF THE
... The additive system for naming inorganic coordination compounds regards a compound as a combination of a central atom, usually that of a metal, to which a surrounding array of other atoms or groups of atoms is attached, each of which is called a ligand. A coordination entity may be a neutral molecul ...
... The additive system for naming inorganic coordination compounds regards a compound as a combination of a central atom, usually that of a metal, to which a surrounding array of other atoms or groups of atoms is attached, each of which is called a ligand. A coordination entity may be a neutral molecul ...
Document
... the first series of the f-block and are called the inner transition elements These elements are frequently called “rare earths,” a name of historical origin but a clear misnomer because several are not rare at all For many uses, the lanthanides do not have to be separated from one another. A mixture ...
... the first series of the f-block and are called the inner transition elements These elements are frequently called “rare earths,” a name of historical origin but a clear misnomer because several are not rare at all For many uses, the lanthanides do not have to be separated from one another. A mixture ...
Profile in Horizon2020 Projects Portal – issue 11
... The few examples discussed might clearly show that sophisticated synthesis may lead to a plethora of new compounds with unusual structures and properties, even for such an obviously well-known class of compounds like the sulfates. This richness might even be increased if various derivatives of the [ ...
... The few examples discussed might clearly show that sophisticated synthesis may lead to a plethora of new compounds with unusual structures and properties, even for such an obviously well-known class of compounds like the sulfates. This richness might even be increased if various derivatives of the [ ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.