Laboratory Exercise: The Electronic Structure of the Hydrogen Atom
... It is found that an electronically excited atom of a given element can relax and emit a photon whose energy matches the energy of the atom’s electronic transition. The collection of photons emitted by a collection of variably excited atoms of a given element can be separated by a prism into the emis ...
... It is found that an electronically excited atom of a given element can relax and emit a photon whose energy matches the energy of the atom’s electronic transition. The collection of photons emitted by a collection of variably excited atoms of a given element can be separated by a prism into the emis ...
L 35 Modern Physics [1]
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
... orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef ...
Name - Red Hook Central Schools
... In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, φ. The kinetic energy of an ejected photo ...
... In actuality, the energy of a photon is never directly proportional to the energy of an ejected electron because the electron must overcome a potential energy barrier (due to a number of quantum and molecular factors). We call this barrier the work function, φ. The kinetic energy of an ejected photo ...
Propagation in the Galaxy 2: electrons, positrons, antiprotons
... Production of electrons and positrons in the Galaxy depends on the models of cosmic ray containment in the Galaxy. The classical model is called the leaky box model where the cosmic rays move with certain mean free path and have probability p to escape from the Galaxy at every step. The models incl ...
... Production of electrons and positrons in the Galaxy depends on the models of cosmic ray containment in the Galaxy. The classical model is called the leaky box model where the cosmic rays move with certain mean free path and have probability p to escape from the Galaxy at every step. The models incl ...
Name: Period : ______ Chemistry – Chapter 13 – Electrons in
... 4. Write the incorrect electron configuration for Chromium. Also write the orbital notation for this configuration. 5. Write the correct electron configuration for Chromium. Also write the orbital notation for this configuration. 6. Write the incorrect electron configuration for Copper. Also write t ...
... 4. Write the incorrect electron configuration for Chromium. Also write the orbital notation for this configuration. 5. Write the correct electron configuration for Chromium. Also write the orbital notation for this configuration. 6. Write the incorrect electron configuration for Copper. Also write t ...
Alessandro Bettini Introduction to Elementary Particle Physics
... B field of 3.3 T supplied by dipoles, what is the maximum energy that can be stored ? ...
... B field of 3.3 T supplied by dipoles, what is the maximum energy that can be stored ? ...
Pure Substances and Mixtures
... • Two types of pure substances: • Elements – pure substance made of only one kind of atom • Compounds – pure substances made of two or more different kinds of elements joined together – Compounds cannot be separated by physical means – Compounds are joined in definite proportions. ...
... • Two types of pure substances: • Elements – pure substance made of only one kind of atom • Compounds – pure substances made of two or more different kinds of elements joined together – Compounds cannot be separated by physical means – Compounds are joined in definite proportions. ...
Frank-Hertz experiment with Neon
... electric discharges through various low-pressure gasses in evacuated glass tubes had shown that atoms will only emit light (that is, electromagnetic radiation) at certain discrete frequencies. To overcome this difficulty, Niels Bohr proposed in 1913 the now called Bohr model of the atom, which sugge ...
... electric discharges through various low-pressure gasses in evacuated glass tubes had shown that atoms will only emit light (that is, electromagnetic radiation) at certain discrete frequencies. To overcome this difficulty, Niels Bohr proposed in 1913 the now called Bohr model of the atom, which sugge ...
photoelectric effect Work function
... where Wemitter is the work function of the cathode from which the electrons are ejected/emitted. (It is the difference between the photon energy and the work done in liberating the highest occupied energy level.) Clearly for frequencies less than a critical frequency no electrons are liberated, howe ...
... where Wemitter is the work function of the cathode from which the electrons are ejected/emitted. (It is the difference between the photon energy and the work done in liberating the highest occupied energy level.) Clearly for frequencies less than a critical frequency no electrons are liberated, howe ...
Chapter_5
... • These particles, or photons, of light at the high-frequency (or violet) end of the spectrum had greater energy and could therefore dislodge many more electrons. • He found that the energy of a photon of a certain frequency can be calculated by using the equation: Ephoton= h n where h (Planck’s con ...
... • These particles, or photons, of light at the high-frequency (or violet) end of the spectrum had greater energy and could therefore dislodge many more electrons. • He found that the energy of a photon of a certain frequency can be calculated by using the equation: Ephoton= h n where h (Planck’s con ...
Culver City H.S. • AP Chemistry Name Period ___ Date ___/___/___
... Write the equation for the first ionization energy of Be. What is the phase of Be? ____ (g)/(l)/(s) ...
... Write the equation for the first ionization energy of Be. What is the phase of Be? ____ (g)/(l)/(s) ...
Lecture 1 - UW Canvas
... In Compton scattering only some of the energy of the photon is transferred to an electron upon their collision. The electron would recoil and thus absorb energy. The scattered photon would have less energy, and ...
... In Compton scattering only some of the energy of the photon is transferred to an electron upon their collision. The electron would recoil and thus absorb energy. The scattered photon would have less energy, and ...
Chapter 2 - Las Positas College
... so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited electron drops all the way back to its lowest state (leaving the atom in the ground state) then the energy of the photon ...
... so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited electron drops all the way back to its lowest state (leaving the atom in the ground state) then the energy of the photon ...
chapter 3
... describes the motion of electrons in the orbits radiation is only emitted when an electron “jumps” from an outer orbit, e.g. r3 to a more inner orbit, e.g. r2 or r1 Einitial – Efinal = Δ E = h f determines the frequency of this radiation, this frequency has nothing to do with the angular/orbital fre ...
... describes the motion of electrons in the orbits radiation is only emitted when an electron “jumps” from an outer orbit, e.g. r3 to a more inner orbit, e.g. r2 or r1 Einitial – Efinal = Δ E = h f determines the frequency of this radiation, this frequency has nothing to do with the angular/orbital fre ...
Moderne Methoden der Materialcharakterisierung
... Typically X-ray radiation is generated by deceleration of electrons ...
... Typically X-ray radiation is generated by deceleration of electrons ...
Atomic Structure Notes
... where n is an integer, h is Planck’s constant and ν is the frequency of the electromagnetic radiation absorbed or emitted. 2. Energy is in fact quantized and can only occur in discrete units of size hv. Each of these small "packets" of energy is called a quantum (or a photon when we are talking abou ...
... where n is an integer, h is Planck’s constant and ν is the frequency of the electromagnetic radiation absorbed or emitted. 2. Energy is in fact quantized and can only occur in discrete units of size hv. Each of these small "packets" of energy is called a quantum (or a photon when we are talking abou ...
chemistry - cloudfront.net
... READ THE FOLLOWING PARAGRAPH BEFORE YOU START! This worksheet is meant to be a “practice test” to help you prepare for your final exam. Suggestions on how to prepare: I would first order my notes and homework by the dates on them. Then I would look at the topic statement below (the capital letter ph ...
... READ THE FOLLOWING PARAGRAPH BEFORE YOU START! This worksheet is meant to be a “practice test” to help you prepare for your final exam. Suggestions on how to prepare: I would first order my notes and homework by the dates on them. Then I would look at the topic statement below (the capital letter ph ...
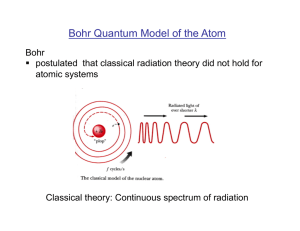
Bohr Quantum Model of the Atom
... levels and orbits (stationary states) § applied Einstein’s concept of the photon to arrive at an expressions for the frequency of the light emitted when electron jumps from one stationary state (i) to another (f) § postulated that the electron orbital momentum is quantized Justification of Bohr’ ...
... levels and orbits (stationary states) § applied Einstein’s concept of the photon to arrive at an expressions for the frequency of the light emitted when electron jumps from one stationary state (i) to another (f) § postulated that the electron orbital momentum is quantized Justification of Bohr’ ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.



![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001689016_1-3e506855e2f70cb00e132a79d00855e2-300x300.png)