Early Modern Physics
... • observed that electrons scattered off of crystals had a diffraction pattern. Readily understood if “matter” particles (with mass) have the same relation ...
... • observed that electrons scattered off of crystals had a diffraction pattern. Readily understood if “matter” particles (with mass) have the same relation ...
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
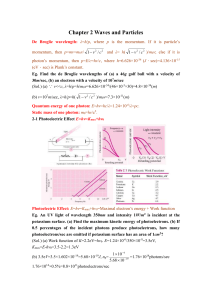
... The shortest wavelength of X-ray due to electron bombard: λmin=1.24×10-6/V, where V is the accelerating voltage. Eg. Find the shortest wavelength present in the radiation from an X-ray machine whose accelerating potential is 50000V. (Sol.) λmin=1.24×10-6/50000=2.5×10-11m=0.025nm 2-2 Bragg’s Reflecti ...
... The shortest wavelength of X-ray due to electron bombard: λmin=1.24×10-6/V, where V is the accelerating voltage. Eg. Find the shortest wavelength present in the radiation from an X-ray machine whose accelerating potential is 50000V. (Sol.) λmin=1.24×10-6/50000=2.5×10-11m=0.025nm 2-2 Bragg’s Reflecti ...
kinetic energy of photoelectrons (eV)
... •Planck’s theory was revolutionary because it proposed that an object cannot vibrate with any amount of energy – only specific amounts. •Whereas, classical theory said energy could be sent out in continuous streams of waves ...
... •Planck’s theory was revolutionary because it proposed that an object cannot vibrate with any amount of energy – only specific amounts. •Whereas, classical theory said energy could be sent out in continuous streams of waves ...
Chapter 4: Arrangement of Electrons in Atoms
... 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, etc.) Figure 16 on page 111. 3. There are exceptions to the Aufbau Principle. They are Cr and down, and Cu and down. Cr: [Ar] 4s13d5; Cu: [Ar] 4s13d10. C. The Pauli Exclusion Principle 1. The Pauli Exclusion Principle states that no two electrons may have the ...
... 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, etc.) Figure 16 on page 111. 3. There are exceptions to the Aufbau Principle. They are Cr and down, and Cu and down. Cr: [Ar] 4s13d5; Cu: [Ar] 4s13d10. C. The Pauli Exclusion Principle 1. The Pauli Exclusion Principle states that no two electrons may have the ...
Electronic structure (download)
... the illusive electron We can predict the motion of a ball; But not an electron: problems locating small objects ...
... the illusive electron We can predict the motion of a ball; But not an electron: problems locating small objects ...
Problem Set 11: Chemistry Graduate Quantum I Physics 6572
... into the nucleus costs a Coulomb energy (as in (Zq)2 /R) of −aC Z 2 /A1/3 ; this Coulomb energy is why there are more neutrons than protons in heavy nuclei. In addition, there are two quantum terms. The first is the Pauli term, which is related to exercise (3); if the number of neutrons N is differe ...
... into the nucleus costs a Coulomb energy (as in (Zq)2 /R) of −aC Z 2 /A1/3 ; this Coulomb energy is why there are more neutrons than protons in heavy nuclei. In addition, there are two quantum terms. The first is the Pauli term, which is related to exercise (3); if the number of neutrons N is differe ...
Arrangement of Electrons in Atoms
... The number of wave cycles that pass a given point per unit of time. Units of frequency are cycles/second or hertz. ...
... The number of wave cycles that pass a given point per unit of time. Units of frequency are cycles/second or hertz. ...
Exam Review - hrsbstaff.ednet.ns.ca
... Rutherford's observation that a gold foil scatters some alpha particle through angles greater than 90º enabled him to conclude that a) all atoms are electrically neutral. b) the nucleus of the atom contains the positive charge. c) an electron has a very small mass. d) electrons are a part of all mat ...
... Rutherford's observation that a gold foil scatters some alpha particle through angles greater than 90º enabled him to conclude that a) all atoms are electrically neutral. b) the nucleus of the atom contains the positive charge. c) an electron has a very small mass. d) electrons are a part of all mat ...
Molecular Statistics
... another, there will be no relation among the coordinates of the two atoms. On the other hand, when the two atoms are bound, the displacement of each other is coupled to the other. The result is to give three translational, one vibrational, and two rotational degrees of freedom for the ...
... another, there will be no relation among the coordinates of the two atoms. On the other hand, when the two atoms are bound, the displacement of each other is coupled to the other. The result is to give three translational, one vibrational, and two rotational degrees of freedom for the ...
Chapter 6 Outline full
... Heisenberg’s uncertainty principle: We cannot determine the exact position, direction of motion, and speed of subatomic particles simultaneously. ...
... Heisenberg’s uncertainty principle: We cannot determine the exact position, direction of motion, and speed of subatomic particles simultaneously. ...
Topic 7: Atomic and nuclear physics 7.1 The atom
... 1. Electrons that are accelerating (and here we have centripetal acceleration) are known to radiate light energy. The electrons in Rutherford’s model should, therefore lose energy and spiral into the nucleus in a matter of nanoseconds. Rutherford’s model, then, cannot explain why stable atoms exist. ...
... 1. Electrons that are accelerating (and here we have centripetal acceleration) are known to radiate light energy. The electrons in Rutherford’s model should, therefore lose energy and spiral into the nucleus in a matter of nanoseconds. Rutherford’s model, then, cannot explain why stable atoms exist. ...
Radiation
... Electromagnetic Radiation •EM sometimes act like particles, sometimes like waves •Particle concept explains ...
... Electromagnetic Radiation •EM sometimes act like particles, sometimes like waves •Particle concept explains ...
Electromagnetic Spectrum activity
... When electrons do this, energy is emitted in the form of light. Each transition or drop has a certain energy associated with it and produces light of a certain frequency from E=h. When electrons drop back to the n=1 level then E and are high and light emitted is in the ultra violet region of the ...
... When electrons do this, energy is emitted in the form of light. Each transition or drop has a certain energy associated with it and produces light of a certain frequency from E=h. When electrons drop back to the n=1 level then E and are high and light emitted is in the ultra violet region of the ...
6.7 – Ionic Compounds
... and Group 3A will tend to lose 3 valence electrons and become 3+ (Al3+). Transition metals will often have different charges. Anion – A nonmetal that has gained valence electrons, and is a negatively charged ion. Halogens will gain 1 valence electron and become - (F-), the oxygen family will tend to ...
... and Group 3A will tend to lose 3 valence electrons and become 3+ (Al3+). Transition metals will often have different charges. Anion – A nonmetal that has gained valence electrons, and is a negatively charged ion. Halogens will gain 1 valence electron and become - (F-), the oxygen family will tend to ...
- Synchrotron emission: A brief history - Examples
... - Discovers a steady source of radio emission from the sky that varied on a cycle of 23 hours 56 minutes (sidereal day) - Comparing his observations to optical astronomical maps, he realized that this radiation was coming from the Milky Way and was strongest towards Sagittarius, the center of our G ...
... - Discovers a steady source of radio emission from the sky that varied on a cycle of 23 hours 56 minutes (sidereal day) - Comparing his observations to optical astronomical maps, he realized that this radiation was coming from the Milky Way and was strongest towards Sagittarius, the center of our G ...
WP1
... the photon used must have a small wavelength (a large wavelength would not have enough resolution to locate the electron, hence there would be an uncertainty in the electron’s position Dx). But a small wavelength for a photon, means a large energy and hence momentum (p = E/c), which gives the electr ...
... the photon used must have a small wavelength (a large wavelength would not have enough resolution to locate the electron, hence there would be an uncertainty in the electron’s position Dx). But a small wavelength for a photon, means a large energy and hence momentum (p = E/c), which gives the electr ...
Astro 10-Lecture 5: Heat, Light and the Structure of Atoms
... • Atoms have few discrete energy levels. – Collisions can cause photons to jump to a higher state. Again, the hotter it is, the bigger a jump the electron can make and the more photons can be made. – Only photons of energy corresponding to the difference in levels can be emitted. ...
... • Atoms have few discrete energy levels. – Collisions can cause photons to jump to a higher state. Again, the hotter it is, the bigger a jump the electron can make and the more photons can be made. – Only photons of energy corresponding to the difference in levels can be emitted. ...
Chapter 5
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
Review for second exam:
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
... Finding the number of protons, neutrons, and electrons from the symbol for an ion Method for finding electron configurations for metal cations (write configuration for the atom, then remove electrons from the highest n, or highest l (for orbitals with same n) to get correct charge) Trends in ion siz ...
Welcome to the Vanderbilt Center for Radiation Oncology
... The electron is deflected from its path by its attraction to the nucleus – This produces an ...
... The electron is deflected from its path by its attraction to the nucleus – This produces an ...
Quantum Mechanics Problem Set
... (a) The hydrogen atom 1s and 2s orbitals have the same overall spherical shape, but the 2s orbital has a larger radial extension and one more node than the 1s orbital. (b) A single 2p orbital is directional in that its electron density is concentrated along one of the three Cartesian axes of the ato ...
... (a) The hydrogen atom 1s and 2s orbitals have the same overall spherical shape, but the 2s orbital has a larger radial extension and one more node than the 1s orbital. (b) A single 2p orbital is directional in that its electron density is concentrated along one of the three Cartesian axes of the ato ...
Chem Ch. 4.4
... • How, you may ask, is it possible for an electron to come from a nucleus?? • Scientists believe that neutrons are actually composed of 2 particles - an electron and a proton. • In beta decay, the electron is emitted and the proton is left, increasing the atomic number. ...
... • How, you may ask, is it possible for an electron to come from a nucleus?? • Scientists believe that neutrons are actually composed of 2 particles - an electron and a proton. • In beta decay, the electron is emitted and the proton is left, increasing the atomic number. ...
Quantum Mechanical Model
... Schrodinger (1926): wrote a mathematical equation to describe an atom using all the information available to date. This modern theory called the Quantum Mechanical Theory describes the positions of the electrons ...
... Schrodinger (1926): wrote a mathematical equation to describe an atom using all the information available to date. This modern theory called the Quantum Mechanical Theory describes the positions of the electrons ...
Atomic Structure and Periodicity
... waves by wavelength, frequency, and energy l – wavelength- the length from a point on a wave to a corresponding point later in the wave. n – frequency- the number of times a full wave cycle passes by a reference point in one second. ...
... waves by wavelength, frequency, and energy l – wavelength- the length from a point on a wave to a corresponding point later in the wave. n – frequency- the number of times a full wave cycle passes by a reference point in one second. ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.