Atomic Structure Lecture 7 - Introduction Lecture 7
... The wave function, !, is also called an atomic orbital. • There is a different wave function for each of the different energy states that an electron can have in an atom While the wave function, !, has no physical meaning, the square of the wave function, !2, is does. • !2 is called the probability ...
... The wave function, !, is also called an atomic orbital. • There is a different wave function for each of the different energy states that an electron can have in an atom While the wave function, !, has no physical meaning, the square of the wave function, !2, is does. • !2 is called the probability ...
CHE 106 Chapter 6
... Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers n,l, ml and ms. In order to put electrons in the same orbital, they must have different ms numbers = opposite spins. ...
... Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers n,l, ml and ms. In order to put electrons in the same orbital, they must have different ms numbers = opposite spins. ...
Molecular geometry
... standard atomic orbitals; chemical bonds result from an overlap of these orbitals. Molecular orbital theory (MO): An advanced model of chemical bonding in which electrons reside in molecular orbitals delocalized over the entire molecule. In the simplest version, the molecular orbitals are simply l ...
... standard atomic orbitals; chemical bonds result from an overlap of these orbitals. Molecular orbital theory (MO): An advanced model of chemical bonding in which electrons reside in molecular orbitals delocalized over the entire molecule. In the simplest version, the molecular orbitals are simply l ...
Unit 3 Electron Notes
... Only certain frequencies satisfied his mathematical equations, which described the wave properties of electrons. Orbital = 3D region around the nucleus that indicates the probable location of an electron ...
... Only certain frequencies satisfied his mathematical equations, which described the wave properties of electrons. Orbital = 3D region around the nucleus that indicates the probable location of an electron ...
Electronic transitions
... Born-Oppenheimer Approximation is the assumption that the electronic motion and the nuclear motion in molecules can be separated. ...
... Born-Oppenheimer Approximation is the assumption that the electronic motion and the nuclear motion in molecules can be separated. ...
Click here to Ch 06.2 Covalent Bonding_Lewis Structures
... • Noble gas atoms are unreactive because their electron configurations are especially stable. • This stability results from the fact that the noble-gas atoms’ outer s and p orbitals are completely filled by a total of eight electrons. • Other atoms can fill their outermost s and p orbitals by sharin ...
... • Noble gas atoms are unreactive because their electron configurations are especially stable. • This stability results from the fact that the noble-gas atoms’ outer s and p orbitals are completely filled by a total of eight electrons. • Other atoms can fill their outermost s and p orbitals by sharin ...
ENT145/3 Materials Engineering Tutorial 1 (Answer) 1. Why is it
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
Chapter 11 Notes
... macroscopic quantities are enormous compared to the wave behavior; hence, we do not normally associate wave characteristics with common objects. The electron, however, is sufficiently small (it has very little mass) and so the wave characteristics are much more pronounced. Here, we will examine the ...
... macroscopic quantities are enormous compared to the wave behavior; hence, we do not normally associate wave characteristics with common objects. The electron, however, is sufficiently small (it has very little mass) and so the wave characteristics are much more pronounced. Here, we will examine the ...
Section 2 Notes
... overlap each other and change to become one final wave Diffraction: The bending of a wave as it passes by the edge of an object Derived from the wave Particle Duality The Basis for Quantum Theory wave properties of electrons and other very small particles are described mathematically ...
... overlap each other and change to become one final wave Diffraction: The bending of a wave as it passes by the edge of an object Derived from the wave Particle Duality The Basis for Quantum Theory wave properties of electrons and other very small particles are described mathematically ...
Chapter Excerpt
... This list may be constructed by arranging the subshells according to n and l and drawing diagonal arrows as shown below: 1s 2s 2p 3s 3 p 3d 4s 4 p 4d 4f 5s 5 p 5d 5f 5 g 6s 6 p 6d 6f 6g 7s 7 p 7d 7f 7 g 8s 8 p 8d 8f 8 g Drawing electron shell structures Electron shell structures (also called electro ...
... This list may be constructed by arranging the subshells according to n and l and drawing diagonal arrows as shown below: 1s 2s 2p 3s 3 p 3d 4s 4 p 4d 4f 5s 5 p 5d 5f 5 g 6s 6 p 6d 6f 6g 7s 7 p 7d 7f 7 g 8s 8 p 8d 8f 8 g Drawing electron shell structures Electron shell structures (also called electro ...
Syracuse Syllabus
... understanding of math and algebra, including an understanding of decimals, exponents, logarithms, quadratics, and algebraic equations, is essential to success in this course (calculus is not required). You should not be taking remedial algebra concurrently with this course. Topics included are atomi ...
... understanding of math and algebra, including an understanding of decimals, exponents, logarithms, quadratics, and algebraic equations, is essential to success in this course (calculus is not required). You should not be taking remedial algebra concurrently with this course. Topics included are atomi ...
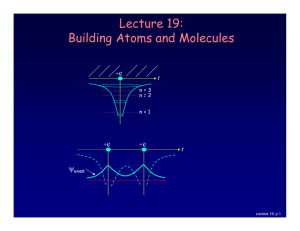
Lecture 19: Building Atoms and Molecules
... Let’s consider what happens when there is more than one electron: • 2 electrons (two neutral H atoms): Both electrons occupy the bonding state (with different ms). This is neutral H2. • 4 electrons (two neutral He atoms). Two electron must be in the anti-bonding state. The repulsive force cancels th ...
... Let’s consider what happens when there is more than one electron: • 2 electrons (two neutral H atoms): Both electrons occupy the bonding state (with different ms). This is neutral H2. • 4 electrons (two neutral He atoms). Two electron must be in the anti-bonding state. The repulsive force cancels th ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.