Electron shell contributions to gamma
... exceeding a few per cent [4]. In addition, the wavefunctions (orbitals) of the innermost s and p electrons in heavier noble gas atoms (i.e. Ar and beyond) extend to significantly larger momentum regions, namely, greater than the 10 au cut-off momentum in the present study. As a result, it is the inn ...
... exceeding a few per cent [4]. In addition, the wavefunctions (orbitals) of the innermost s and p electrons in heavier noble gas atoms (i.e. Ar and beyond) extend to significantly larger momentum regions, namely, greater than the 10 au cut-off momentum in the present study. As a result, it is the inn ...
Group Theory - gozips.uakron.edu
... The procedure described below is sometimes called the “basis function generating machine.” It enables us to start with any function defined in the relevant coordinate space and project out a function that transforms as any desired irreducible representation of the applicable group. (If the starting ...
... The procedure described below is sometimes called the “basis function generating machine.” It enables us to start with any function defined in the relevant coordinate space and project out a function that transforms as any desired irreducible representation of the applicable group. (If the starting ...
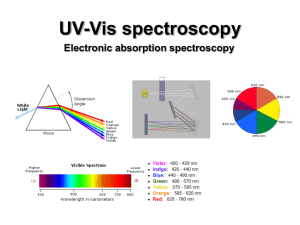
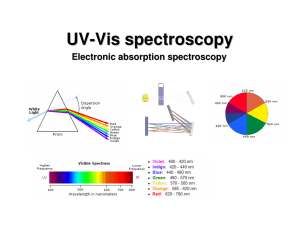
UV-Vis (electronic) spectroscopy
... Absorptions of UV-vis photons by molecule results in electronic excitation of molecule with chromophore. chromophore Any group of atoms that absorbs light whether or not a color is thereby produced. ...
... Absorptions of UV-vis photons by molecule results in electronic excitation of molecule with chromophore. chromophore Any group of atoms that absorbs light whether or not a color is thereby produced. ...
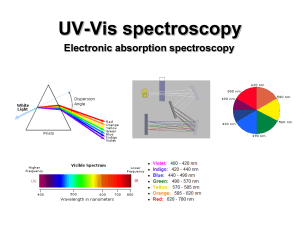
UV-Vis (electronic) spectroscopy
... Absorptions of UV-vis photons by molecule results in electronic excitation of molecule with chromophore. chromophore Any group of atoms that absorbs light whether or not a color is thereby produced. ...
... Absorptions of UV-vis photons by molecule results in electronic excitation of molecule with chromophore. chromophore Any group of atoms that absorbs light whether or not a color is thereby produced. ...
Graph theory in chemistry
... Electrons, e-, surround the nucleus in various energy states, with the outermost state being occupied known as the valence shell. ...
... Electrons, e-, surround the nucleus in various energy states, with the outermost state being occupied known as the valence shell. ...
S3 Numeracy Booklets – Atomic Structure
... What is the difference between an atom of chlorine-35 and an atom of ...
... What is the difference between an atom of chlorine-35 and an atom of ...
∙ ∙B x
... Carbon atoms are hexagonally arranged in flat, parallel .................. Each carbon atom is bonded to ........... other atoms in its layer by .................... bonding. Three carbon electrons form three σ/bonds, the fourth one is called ............................. electron. It may move thr ...
... Carbon atoms are hexagonally arranged in flat, parallel .................. Each carbon atom is bonded to ........... other atoms in its layer by .................... bonding. Three carbon electrons form three σ/bonds, the fourth one is called ............................. electron. It may move thr ...
∙ ∙B x
... Carbon atoms are hexagonally arranged in flat, parallel .................. Each carbon atom is bonded to ........... other atoms in its layer by .................... bonding. Three carbon electrons form three σ/bonds, the fourth one is called ............................. electron. It may move thr ...
... Carbon atoms are hexagonally arranged in flat, parallel .................. Each carbon atom is bonded to ........... other atoms in its layer by .................... bonding. Three carbon electrons form three σ/bonds, the fourth one is called ............................. electron. It may move thr ...
Biol 1020 Ch. 2 Chemistry
... Oxidation-Reduction Reactions Are Common in Biological Systems example: rusting ...
... Oxidation-Reduction Reactions Are Common in Biological Systems example: rusting ...
5.1 Revising the Atomic Model
... the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus of an atom. Each energy sublevel corresponds to one or more orbitals of different shapes, which describe where the electron is likely to be ...
... the allowed energies an electron can have and how likely it is to find the electron in various locations around the nucleus of an atom. Each energy sublevel corresponds to one or more orbitals of different shapes, which describe where the electron is likely to be ...
Hund`s multiplicity rule: From atoms to quantum dots
... singlet and triplet excited states can be explored by calculations within the framework of the mean-field approximation, and, surprisingly, without the need of introducing the angular electronic correlation. Moreover, our calculations have shown that the triplet state of the QD is lower in energy th ...
... singlet and triplet excited states can be explored by calculations within the framework of the mean-field approximation, and, surprisingly, without the need of introducing the angular electronic correlation. Moreover, our calculations have shown that the triplet state of the QD is lower in energy th ...
Application of Hartree-Fock Method for Modeling of Bioactive
... two (2) compounds of a set of twelve (12) test predicted as of high activity were proposed [19]. Artemisinin derivatives with antimalarial activity against Plasmodium falciparum, which is resistant to mefloquine, were studied using quantum chemical methods (HF/6-31G*) and the partial least-squares ( ...
... two (2) compounds of a set of twelve (12) test predicted as of high activity were proposed [19]. Artemisinin derivatives with antimalarial activity against Plasmodium falciparum, which is resistant to mefloquine, were studied using quantum chemical methods (HF/6-31G*) and the partial least-squares ( ...
Unit 4 Notes
... first. D. According to the Pauli exclusion principle, an atomic orbital may hold at most ...
... first. D. According to the Pauli exclusion principle, an atomic orbital may hold at most ...
Periodic Properties of the Elements Effective Nuclear Charge, Zeff
... nucleus thus easier to remove. We see some exceptions however. For example, IE1 of N is greater than IE1 of O. Why? Half-filled p-sublevel for N is more stable than the partially filled p-sublevel for O. In N, we have no e– - e– repulsive pairing energy since all p-orbitals have only 1 e–. In O we h ...
... nucleus thus easier to remove. We see some exceptions however. For example, IE1 of N is greater than IE1 of O. Why? Half-filled p-sublevel for N is more stable than the partially filled p-sublevel for O. In N, we have no e– - e– repulsive pairing energy since all p-orbitals have only 1 e–. In O we h ...
Spectroscopy of Non-Heme Iron Thiolate Complexes: Insight into the
... 3dπ f 3dσ ligand-field transitions in this species occur at higher energies (>15000 cm-1), reflecting its nearoctahedral symmetry. The Fe 3dxz,yz f Fe 3dxy (dπ f dπ) transition occurs in the near-IR and probes the FeIII−S π-donor bond; this transition reveals vibronic structure that reflects the str ...
... 3dπ f 3dσ ligand-field transitions in this species occur at higher energies (>15000 cm-1), reflecting its nearoctahedral symmetry. The Fe 3dxz,yz f Fe 3dxy (dπ f dπ) transition occurs in the near-IR and probes the FeIII−S π-donor bond; this transition reveals vibronic structure that reflects the str ...
Document
... • The Pauli exclusion principle states that a maximum of two electrons can occupy a single orbital, but only if the electrons have opposite spins. • Hund’s rule states that single electrons with the same spin must occupy each equal-energy orbital before additional electrons with opposite spins can o ...
... • The Pauli exclusion principle states that a maximum of two electrons can occupy a single orbital, but only if the electrons have opposite spins. • Hund’s rule states that single electrons with the same spin must occupy each equal-energy orbital before additional electrons with opposite spins can o ...
CHEM 481. Assignment 0. Review of General Chemistry. Answers
... 23. What is a photon? Explain how the photoelectric effect implies the existence of photons. A photon expresses the energy of a wave-packet, hν, the smallest energy unit for a given electromagnetic wave. If the energy of the wavepacket is as large as or larger than the binding energy of the metal, a ...
... 23. What is a photon? Explain how the photoelectric effect implies the existence of photons. A photon expresses the energy of a wave-packet, hν, the smallest energy unit for a given electromagnetic wave. If the energy of the wavepacket is as large as or larger than the binding energy of the metal, a ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.