Chemistry 11 Exam 1 Spring 2006 When answering questions be
... When atoms form covalent bonds each can provide one electron. In molecular orbitals each atom possesses an atomic orbital. These two atomic orbitals combine to form new two new orbitals called molecular orbitals. In the MOs one is lower in energy than the atomic orbitals and the other is higher in e ...
... When atoms form covalent bonds each can provide one electron. In molecular orbitals each atom possesses an atomic orbital. These two atomic orbitals combine to form new two new orbitals called molecular orbitals. In the MOs one is lower in energy than the atomic orbitals and the other is higher in e ...
Chemical Bonding - The Free Information Society
... actually required to break the chemical bond; the difference is the very small zero-point energy as explained inFig. 3. Bond energies are usually determined indirectly from thermodynamic data, but there are two main experimental ways of measuring them directly: 1. The direct thermochemical method in ...
... actually required to break the chemical bond; the difference is the very small zero-point energy as explained inFig. 3. Bond energies are usually determined indirectly from thermodynamic data, but there are two main experimental ways of measuring them directly: 1. The direct thermochemical method in ...
Chapter 5 Equations for Wave Function
... The following figure shows the labeling of sigma functions and hybrid orbitals we will use in carrying out these tasks. Taking the pendant atom sigma functions as the basis set, we can readily show that the reducible representation for the SALCs in D3h is Γσ= 2A1’ +A”2 +E.’ ...
... The following figure shows the labeling of sigma functions and hybrid orbitals we will use in carrying out these tasks. Taking the pendant atom sigma functions as the basis set, we can readily show that the reducible representation for the SALCs in D3h is Γσ= 2A1’ +A”2 +E.’ ...
Revision Y12 Chemistry PLC
... (iii) iii) the number of orbitals making up s-, p- and d-sub-shells, and the number of electrons that can fill s-, p- and d-sub-shells (c) filling of orbitals: (i) ...
... (iii) iii) the number of orbitals making up s-, p- and d-sub-shells, and the number of electrons that can fill s-, p- and d-sub-shells (c) filling of orbitals: (i) ...
QUESTION BANK ON ATOMIC STRUCTURE-3.pmd
... Q68. The probability of finding an electron in the px orbital is (A) zero at nucleus (B) the same on all the sides around nucleus (C) zero on the z-axis (D) maximum on the two opposite sides of the nucleus along the x-axis Q69. The spin of the electron (A) increases the angular momentum (B) decrease ...
... Q68. The probability of finding an electron in the px orbital is (A) zero at nucleus (B) the same on all the sides around nucleus (C) zero on the z-axis (D) maximum on the two opposite sides of the nucleus along the x-axis Q69. The spin of the electron (A) increases the angular momentum (B) decrease ...
Document
... a) 25 g lead (density = 11.3 g/cm3) b) 15 cm3 aluminum (density = 2.70 g/cm3) c) 4.0 cm3 chromium (density = 7.20 g/cm3) ...
... a) 25 g lead (density = 11.3 g/cm3) b) 15 cm3 aluminum (density = 2.70 g/cm3) c) 4.0 cm3 chromium (density = 7.20 g/cm3) ...
bond
... • The Pauli exclusion principle: only two electrons can occupy one atomic orbital and the two electrons have opposite spin • Hund’s rule: electrons will occupy empty degenerated orbitals before pairing up in the same orbital Electrons in inner shells (those below the outermost shell) are called core ...
... • The Pauli exclusion principle: only two electrons can occupy one atomic orbital and the two electrons have opposite spin • Hund’s rule: electrons will occupy empty degenerated orbitals before pairing up in the same orbital Electrons in inner shells (those below the outermost shell) are called core ...
PIB and HH - Unit 4 - Chemical Names and Formulas
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
Electronic structure and reactivity analysis of some TTF
... It has also been incorporated in a number of macrocyclic systems for use as molecular sensors, enzyme biosensors, switches, wires and shuttles, exploiting the inherent electron donor properties [3]. The main purposes of theoretical chemistry based on the adequate knowledge of the general behavior of ...
... It has also been incorporated in a number of macrocyclic systems for use as molecular sensors, enzyme biosensors, switches, wires and shuttles, exploiting the inherent electron donor properties [3]. The main purposes of theoretical chemistry based on the adequate knowledge of the general behavior of ...
Coordination chemistry with selected topics in bioinorganic chemistry
... intermolecular interactions. The interactions: chemical bonds and intermolecular forces will be defined and sorted by their energies to illustrate the relative strength of coordination bond on the energy scale. The qualitative description of the valence bond and molecular orbital theories is present ...
... intermolecular interactions. The interactions: chemical bonds and intermolecular forces will be defined and sorted by their energies to illustrate the relative strength of coordination bond on the energy scale. The qualitative description of the valence bond and molecular orbital theories is present ...
computational chemistry
... The reader is advised to start with this book and to then delve further into the computational literature pertaining to his or her speci®c work. It is impossible to reference all relevant works in a book such as this. The bibliography included at the end of each chapter primarily lists textbooks and ...
... The reader is advised to start with this book and to then delve further into the computational literature pertaining to his or her speci®c work. It is impossible to reference all relevant works in a book such as this. The bibliography included at the end of each chapter primarily lists textbooks and ...
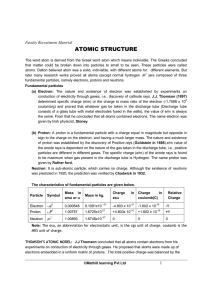
atomic structure
... equal to sum of masses of all the protons and neutrons simply. Most of the elements in nature exist as mixture of isotopes. For these elements atomic mass is calculated on the bases of their abundance in nature and atomic masses of individual members. Isotopes: Atoms of same element which have same ...
... equal to sum of masses of all the protons and neutrons simply. Most of the elements in nature exist as mixture of isotopes. For these elements atomic mass is calculated on the bases of their abundance in nature and atomic masses of individual members. Isotopes: Atoms of same element which have same ...
AP Atomics Class Packet Unit 2 - Ms. Drury`s Flipped Chemistry
... The rules that you have been applying in order to determine the electronic configuration of an atom are: A. Lowest energy orbitals are filled first. THE AUFBAU PRINCIPLE. B. Orbitals can only contain a maximum of two electrons and when two electrons enter the same orbital they must have opposite spi ...
... The rules that you have been applying in order to determine the electronic configuration of an atom are: A. Lowest energy orbitals are filled first. THE AUFBAU PRINCIPLE. B. Orbitals can only contain a maximum of two electrons and when two electrons enter the same orbital they must have opposite spi ...
AP Atomics Class Packet Unit 2 - Ms. Drury`s Flipped Chemistry
... The rules that you have been applying in order to determine the electronic configuration of an atom are: A. Lowest energy orbitals are filled first. THE AUFBAU PRINCIPLE. B. Orbitals can only contain a maximum of two electrons and when two electrons enter the same orbital they must have opposite spi ...
... The rules that you have been applying in order to determine the electronic configuration of an atom are: A. Lowest energy orbitals are filled first. THE AUFBAU PRINCIPLE. B. Orbitals can only contain a maximum of two electrons and when two electrons enter the same orbital they must have opposite spi ...
Chapter 3
... than 1 angstrom (Å), comment on the magnitude of this uncertainty compared to the size of the atom. Strategy The uncertainty in the velocity, 1 percent of 5×106 m/s, is Δu. Calculate Δx and compare it with the diameter of they hydrogen atom. ...
... than 1 angstrom (Å), comment on the magnitude of this uncertainty compared to the size of the atom. Strategy The uncertainty in the velocity, 1 percent of 5×106 m/s, is Δu. Calculate Δx and compare it with the diameter of they hydrogen atom. ...
Lecture 8 Gaussian basis sets CHEM6085: Density Functional Theory
... 3) Substitute the expression for the basis set expansion of a molecular orbital into the Schrödinger equation for the Kohn-Sham orbitals and derive a matrix representation of the Schrödinger equation, involving the “matrix elements” of the Kohn-Sham Hamiltonian and the overlap matrix of the basis fu ...
... 3) Substitute the expression for the basis set expansion of a molecular orbital into the Schrödinger equation for the Kohn-Sham orbitals and derive a matrix representation of the Schrödinger equation, involving the “matrix elements” of the Kohn-Sham Hamiltonian and the overlap matrix of the basis fu ...
Molecular orbital

In chemistry, a molecular orbital (or MO) is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term orbital was introduced by Robert S. Mulliken in 1932 as an abbreviation for one-electron orbital wave function. At an elementary level, it is used to describe the region of space in which the function has a significant amplitude. Molecular orbitals are usually constructed by combining atomic orbitals or hybrid orbitals from each atom of the molecule, or other molecular orbitals from groups of atoms. They can be quantitatively calculated using the Hartree–Fock or self-consistent field (SCF) methods.