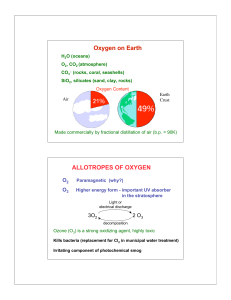
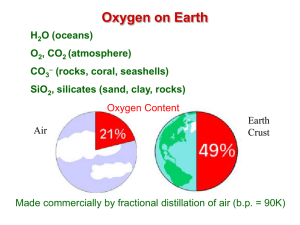
Campbell Biology in Focus (Urry) Chapter 2 The Chemical Context
... 57) Which type of bond must be broken for water to vaporize? A) ionic bonds B) both hydrogen bonds and ionic bonds C) polar covalent bonds D) hydrogen bonds E) both polar covalent bonds and hydrogen bonds 58) Temperature usually increases when water condenses. Which behavior of water is most directl ...
... 57) Which type of bond must be broken for water to vaporize? A) ionic bonds B) both hydrogen bonds and ionic bonds C) polar covalent bonds D) hydrogen bonds E) both polar covalent bonds and hydrogen bonds 58) Temperature usually increases when water condenses. Which behavior of water is most directl ...
the Main-Group Metals - McQuarrie General Chemistry
... polished aluminum has a bright, silvery appearance, but weathered aluminum has a dull tarnish because of the aluminum oxide coating. Structural alloys of aluminum for aircraft, automobiles, and some bicycles contain silicon, copper, magnesium, and other metals, which increase the strength and stiffn ...
... polished aluminum has a bright, silvery appearance, but weathered aluminum has a dull tarnish because of the aluminum oxide coating. Structural alloys of aluminum for aircraft, automobiles, and some bicycles contain silicon, copper, magnesium, and other metals, which increase the strength and stiffn ...
Atoms and Molecules
... • Every atom has a characteristic total number of covalent bonds that it can form - an atom’s valence. • The valence of hydrogen is 1. • Oxygen is 2. • Nitrogen is 3. • Carbon is 4. • Phosphorus should have a valence of 3, based on its three unpaired electrons, but in biological molecules it genera ...
... • Every atom has a characteristic total number of covalent bonds that it can form - an atom’s valence. • The valence of hydrogen is 1. • Oxygen is 2. • Nitrogen is 3. • Carbon is 4. • Phosphorus should have a valence of 3, based on its three unpaired electrons, but in biological molecules it genera ...
401
... a reasonable speed, we can not only predict chemistry very accurately but also simulate chemical phenomena as precisely as or even more precisely than is possible through experiments. This has long been a dream of many scientists since the birth of the SE in 1926.1 In 2000, a breakthrough was initia ...
... a reasonable speed, we can not only predict chemistry very accurately but also simulate chemical phenomena as precisely as or even more precisely than is possible through experiments. This has long been a dream of many scientists since the birth of the SE in 1926.1 In 2000, a breakthrough was initia ...
Name #_____
... A) Metals lose electrons to form anions and nonmetals gain electrons to form cations. B) Metals lose electrons to form cations and nonmetals gain electrons to form anions. C) Metals gain electrons to form anions and nonmetals lose electrons to form cations. D) Metals gain electrons to form cations a ...
... A) Metals lose electrons to form anions and nonmetals gain electrons to form cations. B) Metals lose electrons to form cations and nonmetals gain electrons to form anions. C) Metals gain electrons to form anions and nonmetals lose electrons to form cations. D) Metals gain electrons to form cations a ...
Topic 4
... electrons/electron density between the two (carbon) atoms/OWTTE; (π bond formed by) sideways/parallel overlap; electrons/electron density above and below bond/OWTTE; Marks can be scored from a suitable diagram. ...
... electrons/electron density between the two (carbon) atoms/OWTTE; (π bond formed by) sideways/parallel overlap; electrons/electron density above and below bond/OWTTE; Marks can be scored from a suitable diagram. ...
- Catalyst
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
Study Guide
... 1. Draw the Lewis structure of the bromine atom. 2. How many dots are shown in the Lewis structure for the sulfur atom? 3. What are the two principal types of bonding called? 4. Name the two classes of element which are most likely to form an ionic compound if they are allowed to react with each oth ...
... 1. Draw the Lewis structure of the bromine atom. 2. How many dots are shown in the Lewis structure for the sulfur atom? 3. What are the two principal types of bonding called? 4. Name the two classes of element which are most likely to form an ionic compound if they are allowed to react with each oth ...
Subject Area Assessment Guides
... bonded atoms. In metals valence electrons are not localized to individual atoms but are free to move to temporarily occupy vacant orbitals on adjacent metal atoms. For this reason metals conduct electricity well. When an electron from an atom with low electronegativity (e.g., a metal) is removed by ...
... bonded atoms. In metals valence electrons are not localized to individual atoms but are free to move to temporarily occupy vacant orbitals on adjacent metal atoms. For this reason metals conduct electricity well. When an electron from an atom with low electronegativity (e.g., a metal) is removed by ...
with answers
... (c) Give the electronic configurations of sodium (Na) and nitrogen (N), showing clearly how electrons are distributed between orbitals of the same energy. Na (Z=11): 1s²2s²2p63s N (Z=7): 1s²2s²2px2py2pz (d) Explain why compounds of the formulae Na2O and NH3 form from their respective constituent ele ...
... (c) Give the electronic configurations of sodium (Na) and nitrogen (N), showing clearly how electrons are distributed between orbitals of the same energy. Na (Z=11): 1s²2s²2p63s N (Z=7): 1s²2s²2px2py2pz (d) Explain why compounds of the formulae Na2O and NH3 form from their respective constituent ele ...
Pre-AP Chemistry - Simple Rules for Electron Exchange Simple
... Simple rules for assigning oxidation numbers Tracking electron gain and loss for simple reactions like metals becoming ionized is easy. But in most chemical reactions, it is impossible to simply look at the reactants and products and track the electron exchange. In those cases, we must do some elect ...
... Simple rules for assigning oxidation numbers Tracking electron gain and loss for simple reactions like metals becoming ionized is easy. But in most chemical reactions, it is impossible to simply look at the reactants and products and track the electron exchange. In those cases, we must do some elect ...
Topic 2
... elements were arranged in order of atomic mass (A), they could be placed in horizontal rows such that the elements in the vertical columns had similar properties. – periodic table - tabular arrangement of elements in rows and columns, highlighting the regular repetition of properties of the elements ...
... elements were arranged in order of atomic mass (A), they could be placed in horizontal rows such that the elements in the vertical columns had similar properties. – periodic table - tabular arrangement of elements in rows and columns, highlighting the regular repetition of properties of the elements ...
Click here for the Reaction NOTES Handout
... The two solubility rules that you will use the most are numbers 1 and 4. You should memorize that all group 1A metal and ammonium compounds are soluble. As soon as you see a compound with NH4 1+ Li, Na, K, Rb, Cs, or Fr, you should know that it’s soluble. Also, all nitrates are soluble—look at the e ...
... The two solubility rules that you will use the most are numbers 1 and 4. You should memorize that all group 1A metal and ammonium compounds are soluble. As soon as you see a compound with NH4 1+ Li, Na, K, Rb, Cs, or Fr, you should know that it’s soluble. Also, all nitrates are soluble—look at the e ...
From the Metal to the Molecule
... establishing the rules and limitations of using localized bonding concepts to understand the binding in a large collection of atoms in the metallic state. Since practically all the typical physical properties of a metal depend on the almost free movement of electrons in the solid, the question arise ...
... establishing the rules and limitations of using localized bonding concepts to understand the binding in a large collection of atoms in the metallic state. Since practically all the typical physical properties of a metal depend on the almost free movement of electrons in the solid, the question arise ...
Wizard Test Maker
... (2) propanal (4) water 6856 Which Group 14 element is classified as a metal? (1) carbon (3) silicon (2) germanium (4) tin 6763 An element that has a low first ionization energy and good conductivity of heat and electricity is classified as a (3) nonmetal (1) metal (2) metalloid (4) noble gas 6709 A ...
... (2) propanal (4) water 6856 Which Group 14 element is classified as a metal? (1) carbon (3) silicon (2) germanium (4) tin 6763 An element that has a low first ionization energy and good conductivity of heat and electricity is classified as a (3) nonmetal (1) metal (2) metalloid (4) noble gas 6709 A ...
APS Practice Final 2011
... ____ 102. If you are given the mass of an object in pounds, the time in seconds, and the distance in feet, what must you do before you can calculate the momentum in SI units? a. convert the mass to kilograms c. Both (a) and (b) b. convert the distance to meters d. None of the above ____ 103. Weight ...
... ____ 102. If you are given the mass of an object in pounds, the time in seconds, and the distance in feet, what must you do before you can calculate the momentum in SI units? a. convert the mass to kilograms c. Both (a) and (b) b. convert the distance to meters d. None of the above ____ 103. Weight ...
Activity Series Unit
... 29. Generally speaking, what happens to these species in these reactions? They are gaining electrons from the metals. 30. When looking at electrons, what can be said about the term, reduction? Reduction means the gain of electrons. 31. In these reactions what species is causing reduction? What spec ...
... 29. Generally speaking, what happens to these species in these reactions? They are gaining electrons from the metals. 30. When looking at electrons, what can be said about the term, reduction? Reduction means the gain of electrons. 31. In these reactions what species is causing reduction? What spec ...
Brief Introduction to Superconductivity
... How can we know what are the possible states that a metal electron can occupy? Of interest to us are metals since superconductivity appears in a metallic regime. We will consider crystals. These are periodic arrangements of ions of a certain symmetry (the corners of a cube, for example) with electro ...
... How can we know what are the possible states that a metal electron can occupy? Of interest to us are metals since superconductivity appears in a metallic regime. We will consider crystals. These are periodic arrangements of ions of a certain symmetry (the corners of a cube, for example) with electro ...
Worksheet 2 Due beginning of class Wednesday March 3, 2004
... This is a worksheet and it is assumed that there may be some trial and error in your work. There is no need to erase and recopy work, simply draw and “X” or line thru what you do not want graded, and carry on. Neatness counts. ...
... This is a worksheet and it is assumed that there may be some trial and error in your work. There is no need to erase and recopy work, simply draw and “X” or line thru what you do not want graded, and carry on. Neatness counts. ...
1 - Academics
... In essence, what this means is: a) No particle can travel faster than Planck’s Constant; b) The velocity and the position of an electron can be measured to greater than h/4 significant figures; c) Electrons exhibit wave-particle duality but nothing else does; d) The momentum and the position of a p ...
... In essence, what this means is: a) No particle can travel faster than Planck’s Constant; b) The velocity and the position of an electron can be measured to greater than h/4 significant figures; c) Electrons exhibit wave-particle duality but nothing else does; d) The momentum and the position of a p ...