13.0 Redox Reactions PowerPoint
... nonspontaneous, it should be possible to use a copper pipe to carry hydrochloric acid ...
... nonspontaneous, it should be possible to use a copper pipe to carry hydrochloric acid ...
entropy - KFUPM Faculty List
... Qualitatively, the entropy (S) of a system is a measure of how spread out or how dispersed the system’s energy is. The simplest interpretation of this is how spread out a system’s energy is in space. In other words, for a given system, the greater the volume it occupies, the greater its entropy. ...
... Qualitatively, the entropy (S) of a system is a measure of how spread out or how dispersed the system’s energy is. The simplest interpretation of this is how spread out a system’s energy is in space. In other words, for a given system, the greater the volume it occupies, the greater its entropy. ...
ch20powerpoint
... of arrangements that are available to a system existing in a given state. Probability of occurrence of a particular arrangement(state) depends on the number of ways(microstates) in which it can be arranged. S=k ln W, k= Boltzmann constant=R/Av number=1.38 x 10-23J/K. Un its of S are J/K ...
... of arrangements that are available to a system existing in a given state. Probability of occurrence of a particular arrangement(state) depends on the number of ways(microstates) in which it can be arranged. S=k ln W, k= Boltzmann constant=R/Av number=1.38 x 10-23J/K. Un its of S are J/K ...
Topic 9 - Anderson High School
... • Negative chloride ions are attracted to the positive ions. There they lose electrons and are oxidized to chlorine gas: 2Cl-(l) → Cl2(g) + 2e• Positive sodium ions are attracted to the negative cathode. They gain electrons and are reduced to sodium metal: ...
... • Negative chloride ions are attracted to the positive ions. There they lose electrons and are oxidized to chlorine gas: 2Cl-(l) → Cl2(g) + 2e• Positive sodium ions are attracted to the negative cathode. They gain electrons and are reduced to sodium metal: ...
Refraction and Optical Fibres
... Technology and Mathematics Project funded by the Australian Government Department of Education, Science and Training as a part of the Boosting Innovation in Science, Technology and Mathematics Teaching (BISTMT) Programme.” ...
... Technology and Mathematics Project funded by the Australian Government Department of Education, Science and Training as a part of the Boosting Innovation in Science, Technology and Mathematics Teaching (BISTMT) Programme.” ...
57 estonian national chemistry olympiad
... crystals have signs of blown bubbles, which he explained by water vapor formation. In order to prove his conclusions about the solid product of reaction he passed heated to high temperature fluorine gas above it (2). Produced gases were passed through the aqueous solution of H2F2 (3), on the surface ...
... crystals have signs of blown bubbles, which he explained by water vapor formation. In order to prove his conclusions about the solid product of reaction he passed heated to high temperature fluorine gas above it (2). Produced gases were passed through the aqueous solution of H2F2 (3), on the surface ...
sec chemistry may 2011 marking scheme
... • Light is a form of energy and therefore it increases the rate of reaction / the energy of the reactants increases. • Reaction is photo-catalysed. The green colour of the chlorine gas disappears as the reaction proceeds. ...
... • Light is a form of energy and therefore it increases the rate of reaction / the energy of the reactants increases. • Reaction is photo-catalysed. The green colour of the chlorine gas disappears as the reaction proceeds. ...
Role of Substrate Temperature on the Structural
... prevent the formation of hydroxides. Water is the most convenient oxidizing agent. Methanol and ethanol were the obvious choice because of their volatility and thus facilitating quick transformation of the precursor mist into vapor form, which is an important criterion for obtaining good quality film ...
... prevent the formation of hydroxides. Water is the most convenient oxidizing agent. Methanol and ethanol were the obvious choice because of their volatility and thus facilitating quick transformation of the precursor mist into vapor form, which is an important criterion for obtaining good quality film ...
GCE Getting Started - Edexcel
... Understand the interactions in molecules, such as H2O, liquid NH3 and liquid HF, which give rise to hydrogen bonding. Understand the following anomalous properties of water resulting from hydrogen bonding: i. its relatively high melting temperature and boiling temperature ii. the density of ice comp ...
... Understand the interactions in molecules, such as H2O, liquid NH3 and liquid HF, which give rise to hydrogen bonding. Understand the following anomalous properties of water resulting from hydrogen bonding: i. its relatively high melting temperature and boiling temperature ii. the density of ice comp ...
1 Fundamentals of Chemical Kinetics
... or mol/L are preferred; however, most chemists appear to be ignoring their lead.) For gas phase reactions, the customary units are molecules cm−3 , often written simply cm−3 . The chemical state of a homogeneous system can be described by specifying the concentrations of all the species present and ...
... or mol/L are preferred; however, most chemists appear to be ignoring their lead.) For gas phase reactions, the customary units are molecules cm−3 , often written simply cm−3 . The chemical state of a homogeneous system can be described by specifying the concentrations of all the species present and ...
CHEMISTRY
... Many aldehydes have very strong and characteristic smells. This is the case for cinnamaldehyde, the major component of cinnamon, and vanillin responsible for the characteristic smell of vanilla pods. A molecule of cinnamaldehye contains one benzene ring and also contains one carbon to carbon double ...
... Many aldehydes have very strong and characteristic smells. This is the case for cinnamaldehyde, the major component of cinnamon, and vanillin responsible for the characteristic smell of vanilla pods. A molecule of cinnamaldehye contains one benzene ring and also contains one carbon to carbon double ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Analyze: Our task is to write a net ionic equation for a precipitation reaction, given the names of the reactants present in solution. Plan: We first need to write the chemical formulas of the reactants and products and to determine which product is insoluble. Then we write and balance the molecular ...
... Analyze: Our task is to write a net ionic equation for a precipitation reaction, given the names of the reactants present in solution. Plan: We first need to write the chemical formulas of the reactants and products and to determine which product is insoluble. Then we write and balance the molecular ...
Calculations on the equations reaction
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... reduce any risks. Teachers will assess the readiness level of students to protect everyone in the class. If a student is considered unready, he or she will not be able to participate in the activity. If no other opportunity to participate can be arranged, then the student’s development of hands-on s ...
... reduce any risks. Teachers will assess the readiness level of students to protect everyone in the class. If a student is considered unready, he or she will not be able to participate in the activity. If no other opportunity to participate can be arranged, then the student’s development of hands-on s ...
Chapter 6 Chemical Reactions
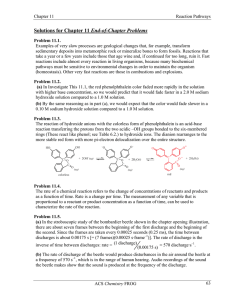
... should speed up as temperature increases. (As we will find in Section 11.6, this is a relatively minor effect compared to the activation energy effect, but even reactions with zero activation energy go faster at higher temperature because of the faster motion.) Problem 11.9. To test whether a substa ...
... should speed up as temperature increases. (As we will find in Section 11.6, this is a relatively minor effect compared to the activation energy effect, but even reactions with zero activation energy go faster at higher temperature because of the faster motion.) Problem 11.9. To test whether a substa ...
California Standards Practice - Student Edition
... 10. The bonding characteristics of carbon allow the formation of many different organic molecules of varied sizes, shapes, and chemical properties and provide the biochemicalbasis of life. As a basis for understanding this concept: a. Students know large molecules (polymers), such as proteins, nucle ...
... 10. The bonding characteristics of carbon allow the formation of many different organic molecules of varied sizes, shapes, and chemical properties and provide the biochemicalbasis of life. As a basis for understanding this concept: a. Students know large molecules (polymers), such as proteins, nucle ...
chem 102 class notes - Louisiana Tech University
... it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains is negligible. Irreversible or complete reactions: Chemical reactions can be considered to have forward and backward reactions. Forward reaction is when reactants combi ...
... it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains is negligible. Irreversible or complete reactions: Chemical reactions can be considered to have forward and backward reactions. Forward reaction is when reactants combi ...
Ch 10 Practice Problems 1. Consider the process A(l) A(s). Which
... C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to zero. C) greater th ...
... C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to zero. C) greater th ...
K c
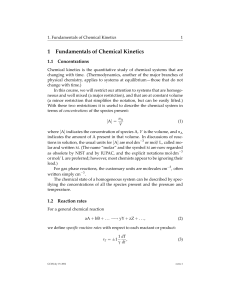
... 1. The concentrations of the reacting species in the condensed phase are expressed in mol/L (M). In the gaseous phase, the concentrations can be expressed in M. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium c ...
... 1. The concentrations of the reacting species in the condensed phase are expressed in mol/L (M). In the gaseous phase, the concentrations can be expressed in M. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium c ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.















![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)