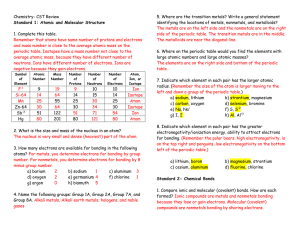
Chemistr.e1a.chapter.4.new2015
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
... • The following reaction that you have seen before in class and the laboratory is neither a precipitation reaction nor an acid-base reaction. Cu (s) + ½ O2 (g) " CuO (s) The reaction above is one where electrons are transferred from one element to another during the reaction. This kind of reaction i ...
Various Types of RXNS
... 5. small chunks of solid sodium are added to water --describe a test to confirm the gaseous product in your reaction 6. hydrobromic acid is added to a solution of potassium hydrogen carbonate --when a gas produced by the reaction is bubbled through limewater, what visible change is expected? 7. aque ...
... 5. small chunks of solid sodium are added to water --describe a test to confirm the gaseous product in your reaction 6. hydrobromic acid is added to a solution of potassium hydrogen carbonate --when a gas produced by the reaction is bubbled through limewater, what visible change is expected? 7. aque ...
Chemical Equilibrium
... Changing the volume of a reactant container changes the concentration of gaseous reactants and therefore their partial pressures Equilibrium position will therefore move The value of Kc or Kp does NOT change Changing pressure by adding more of an inert gas has no effect of the equilibrium position - ...
... Changing the volume of a reactant container changes the concentration of gaseous reactants and therefore their partial pressures Equilibrium position will therefore move The value of Kc or Kp does NOT change Changing pressure by adding more of an inert gas has no effect of the equilibrium position - ...
1442 Final Review
... 35. What is the best definition of a Brønsted-Lowry base? a) electron-pair acceptor b) electron-pair donor *c) proton acceptor d) proton donor e) produces hydroxide ions in aqueous solutions 36. If the concentration of hydroxide ion in a certain solution is 5.8 x 10-3 M, what is the pH of the soluti ...
... 35. What is the best definition of a Brønsted-Lowry base? a) electron-pair acceptor b) electron-pair donor *c) proton acceptor d) proton donor e) produces hydroxide ions in aqueous solutions 36. If the concentration of hydroxide ion in a certain solution is 5.8 x 10-3 M, what is the pH of the soluti ...
Page 1 of 9 Chem 103 Practice Problems: Below is a key for both
... using your calculations using Q. E.g. If you decrease the volume of a gas mixture of A,B and C initially at equilibrium, where: 2A + 4B(g) --> 9C(g), Kp=5. Which reaction direction would be favored? Prove your answer by calculating Qp and comparing it with Kp. What is Kc? Solution: If you decrease t ...
... using your calculations using Q. E.g. If you decrease the volume of a gas mixture of A,B and C initially at equilibrium, where: 2A + 4B(g) --> 9C(g), Kp=5. Which reaction direction would be favored? Prove your answer by calculating Qp and comparing it with Kp. What is Kc? Solution: If you decrease t ...
Power point types of chemical rxn
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
... 1. Elements that form ionic compounds: Magnesium metal reacts with oxygen gas to form magnesium oxide. • 2Mg + O2 2MgO 2. Elements that form covalent compounds: Nitrogen gas and oxygen gas join to form dinitrogen monoxide. • 2N2 + O2 2N2O SYNTHESIS REACTION (iron + sulphur): http://www.youtube.c ...
Final Exam Study Guide Page 1 Quiz
... a. The number of atoms in a mole of an element b. The number of molecules in a mole of a compound c. A and B d. None of the above Use the following equation to answer numbers 9, 10, and 11: Fe + 2H2SO4 → Fe(SO4)2 +2 H2 9. If 2.31g iron reacted with 8.83g sulfuric acid, what is the limiting reactant? ...
... a. The number of atoms in a mole of an element b. The number of molecules in a mole of a compound c. A and B d. None of the above Use the following equation to answer numbers 9, 10, and 11: Fe + 2H2SO4 → Fe(SO4)2 +2 H2 9. If 2.31g iron reacted with 8.83g sulfuric acid, what is the limiting reactant? ...
Reaction Kinetics. The Bromination of Acetone
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
Application of the Purdue Ontology for Pharmaceutical Engineering
... past experience to avoid potential interactions during drug product development. Multiple tools are present to mechanistically predict chemical reactions: however their utility is limited due to the complexity of the domain and the need for explicit information. In this work, the Purdue Ontology for ...
... past experience to avoid potential interactions during drug product development. Multiple tools are present to mechanistically predict chemical reactions: however their utility is limited due to the complexity of the domain and the need for explicit information. In this work, the Purdue Ontology for ...
2007 local exam - American Chemical Society
... 17. A sample of C2H6 gas initially at 50 ˚C and 720 mmHg is heated to 100 ˚C in a container of constant volume. What is the new pressure (in mmHg)? ...
... 17. A sample of C2H6 gas initially at 50 ˚C and 720 mmHg is heated to 100 ˚C in a container of constant volume. What is the new pressure (in mmHg)? ...
Day 72 TYPES OF CHEMICAL REACTIONS
... Release of energy as heat Release of energy as light Change in colour ...
... Release of energy as heat Release of energy as light Change in colour ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.