Chemical Equations and Reactions
... or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mass, the total mass of reactants must equal the tota ...
... or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mass, the total mass of reactants must equal the tota ...
chapter 13 - Humble ISD
... EXAMPLE PROBLEM The following reaction is allowed to go to equilibrium. ...
... EXAMPLE PROBLEM The following reaction is allowed to go to equilibrium. ...
2010 - SAASTA
... There are 4 moles of gaseous reactants and only 2 moles of gaseous product. Lower pressure favours the reaction that loads to an increase in the number of gas molecules, i.e. the reverse reaction. Therefore the equilibrium concentration of nitrogen will increase and the yield of ammonia will decreas ...
... There are 4 moles of gaseous reactants and only 2 moles of gaseous product. Lower pressure favours the reaction that loads to an increase in the number of gas molecules, i.e. the reverse reaction. Therefore the equilibrium concentration of nitrogen will increase and the yield of ammonia will decreas ...
Name: 1) What is the oxidation number of sulfur in H SO ? A)
... In any oxidation-reduction reaction, the total number of electrons gained is A) greater than the total number of electrons lost B) equal to the total number of electrons lost ...
... In any oxidation-reduction reaction, the total number of electrons gained is A) greater than the total number of electrons lost B) equal to the total number of electrons lost ...
physical and chemical change
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
AT 25 °C - University of Bath
... In (a), G(products) < G(reactants). ∆G is negative so the reaction goes forward. The opposite is true for (c). For (b), ∆G would be negative if the system started at either end so the reaction would proceed until point E. If the composition changes, G would increase. Thus, the reaction cannot change ...
... In (a), G(products) < G(reactants). ∆G is negative so the reaction goes forward. The opposite is true for (c). For (b), ∆G would be negative if the system started at either end so the reaction would proceed until point E. If the composition changes, G would increase. Thus, the reaction cannot change ...
IB Definitions
... The first ionization energy is the minimum energy required to remove the outermost electron from each atom in a mole of gaseous atoms Electronegativity is a measure of the ability of an atom to attract a pair of electrons in a covalent bond Define the terms exothermic reaction, endothermic reaction ...
... The first ionization energy is the minimum energy required to remove the outermost electron from each atom in a mole of gaseous atoms Electronegativity is a measure of the ability of an atom to attract a pair of electrons in a covalent bond Define the terms exothermic reaction, endothermic reaction ...
3(aq)
... Reactions that form water • Base: substances that have a bitter taste, and are slippery to the touch (like soap) 1. are also called “alkaline” solutions 2. they are substances that produce hydroxide ions (OH-) when dissolved into water. 3. bases are considered “strong” bases are those that easily d ...
... Reactions that form water • Base: substances that have a bitter taste, and are slippery to the touch (like soap) 1. are also called “alkaline” solutions 2. they are substances that produce hydroxide ions (OH-) when dissolved into water. 3. bases are considered “strong” bases are those that easily d ...
lecture10
... conventions for charge movement (current) were developed before anyone knew about electrons. People just assumed that the things carrying the charges were positive. The assumption was not correct, but the convention stuck. For this reason we have to throw a negative sign into the equation. Ok, now h ...
... conventions for charge movement (current) were developed before anyone knew about electrons. People just assumed that the things carrying the charges were positive. The assumption was not correct, but the convention stuck. For this reason we have to throw a negative sign into the equation. Ok, now h ...
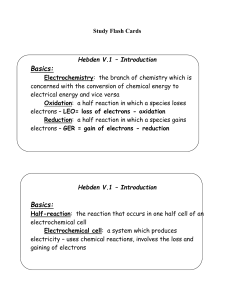
Hebden V.2 – Oxidation Numbers
... 1. Write the oxidation and reduction half reactions with their potentials (from the table) 2. E0cell = E0RED - E0ox 3. If you have to reverse the way the reaction is written to show the reduction or oxidation you must reverse the sign on the E0 4. If E0cell is positive = spontaneous 5. If E0cell is ...
... 1. Write the oxidation and reduction half reactions with their potentials (from the table) 2. E0cell = E0RED - E0ox 3. If you have to reverse the way the reaction is written to show the reduction or oxidation you must reverse the sign on the E0 4. If E0cell is positive = spontaneous 5. If E0cell is ...
Document
... written as separate ions with their own coefficients and phase labels. (Pure solids, liquids, gases, weak electrolytes, and nonelectrolytes written as molecules.) ...
... written as separate ions with their own coefficients and phase labels. (Pure solids, liquids, gases, weak electrolytes, and nonelectrolytes written as molecules.) ...
What is Weathering
... A. What do chemical equations show? B. What are the two parts of a chemical reaction?. C. How is a chemical equation similar to a math equation? How are they different? D. If you collected all the ashes and captured all of the gas from a burning log, what would the total mass be equal to? II. Types ...
... A. What do chemical equations show? B. What are the two parts of a chemical reaction?. C. How is a chemical equation similar to a math equation? How are they different? D. If you collected all the ashes and captured all of the gas from a burning log, what would the total mass be equal to? II. Types ...
DEPARTMENT OF CHEMISTRY
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
General, Organic, and Biological Chemistry
... 72) Which of the following elements does NOT exist as a diatomic molecule? A) hydrogen B) nitrogen C) chlorine D) oxygen E) carbon 73) Choose the best electron-dot structure for OCl2. A) B) C) D) E) 74) The correct name of the compound NCl3 is A) nitrogen chloride. B) trinitrogen chloride C) nitrog ...
... 72) Which of the following elements does NOT exist as a diatomic molecule? A) hydrogen B) nitrogen C) chlorine D) oxygen E) carbon 73) Choose the best electron-dot structure for OCl2. A) B) C) D) E) 74) The correct name of the compound NCl3 is A) nitrogen chloride. B) trinitrogen chloride C) nitrog ...
... molecule - Rate is first order. Bimolecular step - requires two molecules - Rate is second order Termolecular step- requires three molecules - Rate is third order Termolecular steps are almost never heard of because the chances of three molecules coming into contact at the same time are minisc ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.