Reactions in Aqueous Solution
... There are many reasons for carrying out reactions in solution. For a chemical reaction to occur, individual atoms, molecules, or ions must collide, and collisions between two solids, which are not dispersed at the atomic, molecular, or ionic level, do not occur at a significant rate. In addition, wh ...
... There are many reasons for carrying out reactions in solution. For a chemical reaction to occur, individual atoms, molecules, or ions must collide, and collisions between two solids, which are not dispersed at the atomic, molecular, or ionic level, do not occur at a significant rate. In addition, wh ...
Lecture 06 Slides
... 5. The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. ...
... 5. The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. ...
Version 1.6 - Clark Science Center
... located in space?” If all we consider is the nuclei, then the answer is clear: as far apart as they can get, since all nuclei are positively charged, and hence repel each other according to Coulomb’s law, which is at the heart of all chemical understanding: ...
... located in space?” If all we consider is the nuclei, then the answer is clear: as far apart as they can get, since all nuclei are positively charged, and hence repel each other according to Coulomb’s law, which is at the heart of all chemical understanding: ...
Novel Methods and Materials in Development of Liquid Carrier
... unusual perspective: perfluorocarbons (e.g. perflourooctylbromide) were, based on the famous submerged mouse-experiment [5], currently also discussed in medical science as temporary intravenous blood substitutes [6, 7]. Intrinsic oxygen solubility of these oxygen carriers proved to be excitingly hig ...
... unusual perspective: perfluorocarbons (e.g. perflourooctylbromide) were, based on the famous submerged mouse-experiment [5], currently also discussed in medical science as temporary intravenous blood substitutes [6, 7]. Intrinsic oxygen solubility of these oxygen carriers proved to be excitingly hig ...
Chem13-14PrecipABNeut
... write your work when solving the problems in these lessons. A notebook that has graphpaper as its pages will be especially helpful. Choosing a Calculator: As you do problems in these lessons (and assigned homework) that require a calculator, use the same calculator that you will be allowed to use du ...
... write your work when solving the problems in these lessons. A notebook that has graphpaper as its pages will be especially helpful. Choosing a Calculator: As you do problems in these lessons (and assigned homework) that require a calculator, use the same calculator that you will be allowed to use du ...
Fall 2006
... (2 pts) Strontium phosphate reacts with sulfuric acid to form strontium sulfate and phosphoric acid. What is the coefficient for sulfuric acid when the equation is balanced using the lowest, whole-numbered coefficients? a) 1 ...
... (2 pts) Strontium phosphate reacts with sulfuric acid to form strontium sulfate and phosphoric acid. What is the coefficient for sulfuric acid when the equation is balanced using the lowest, whole-numbered coefficients? a) 1 ...
Edita Pusvaškienė
... of the groove and thinner at the edges) the extraction of the less volatile compounds could be problematic because of their low sorption/desorption from the thicker coating layer and could cause peak tailing because of the differences in the coatings thickness. On the other hand, volatile compounds ...
... of the groove and thinner at the edges) the extraction of the less volatile compounds could be problematic because of their low sorption/desorption from the thicker coating layer and could cause peak tailing because of the differences in the coatings thickness. On the other hand, volatile compounds ...
Support Material
... corners of the unit cell and atoms of B are present at the face centres. If one atom of A is missing from its position at the corner, what is the formula of the compound? [Ans. : A7B24] ...
... corners of the unit cell and atoms of B are present at the face centres. If one atom of A is missing from its position at the corner, what is the formula of the compound? [Ans. : A7B24] ...
Probable anticlockwise P-T evolution in extending crust: Hlinsko
... a P-T path with an initial minor increase in pressure followed by cooling. Calculated pseudosections constrain this anticlockwise P-T evolution to the upper part of the andalusite field between 0.36 and 0.40 G P a for temperatures ranging from 570 to 530 -C. A low uHZ0is required to explain the pres ...
... a P-T path with an initial minor increase in pressure followed by cooling. Calculated pseudosections constrain this anticlockwise P-T evolution to the upper part of the andalusite field between 0.36 and 0.40 G P a for temperatures ranging from 570 to 530 -C. A low uHZ0is required to explain the pres ...
Chapter 5 Geochemical Weathering
... ferromagnesian silicates like basalts that dominate in volcanic belts. The kinetics of silicate weathering are slow, with the formation of secondary clays ― hydrated aluminosilcate minerals with high capacity for cation exchange and sorption. Weathering of carbonate rocks proceeds more rapidly than ...
... ferromagnesian silicates like basalts that dominate in volcanic belts. The kinetics of silicate weathering are slow, with the formation of secondary clays ― hydrated aluminosilcate minerals with high capacity for cation exchange and sorption. Weathering of carbonate rocks proceeds more rapidly than ...
CHM 423 Coordination Chemistry
... electron pairs. The presence of empty suitable orbitals in transition metals (Cu, Co, Fe etc) and some compounds (BF3, BeCl2 with empty p-orbital) and ions (H+) of main block elements makes them to act as Lewis acids. However, the chemistry of coordination compounds is restricted to compounds in whi ...
... electron pairs. The presence of empty suitable orbitals in transition metals (Cu, Co, Fe etc) and some compounds (BF3, BeCl2 with empty p-orbital) and ions (H+) of main block elements makes them to act as Lewis acids. However, the chemistry of coordination compounds is restricted to compounds in whi ...
Question Bank for Pre Board Exam(XII Chemistry)
... 65. What makes the crystal of KCl sometimes appear violet?[Hint : F-Centre] 66. Which point defect in ionic crystal does not alter the density of the relevant solid? 67. Name one solid in which both Frenkel and Schottky defects occur. 68. Which type of defects are known as thermodynamic defects? [An ...
... 65. What makes the crystal of KCl sometimes appear violet?[Hint : F-Centre] 66. Which point defect in ionic crystal does not alter the density of the relevant solid? 67. Name one solid in which both Frenkel and Schottky defects occur. 68. Which type of defects are known as thermodynamic defects? [An ...
Tro Chemistry a Molecular Approach, 3E
... law of thermodynamics. The second law—which we examine in more detail throughout this chapter—implies that not only can we not win in an energy transaction, we cannot even break even. For example, consider a rechargeable battery. Suppose that when we use the fully charged battery for some applicatio ...
... law of thermodynamics. The second law—which we examine in more detail throughout this chapter—implies that not only can we not win in an energy transaction, we cannot even break even. For example, consider a rechargeable battery. Suppose that when we use the fully charged battery for some applicatio ...
Arsenic behaviour in subsurface hydrogeochemical systems
... the EQ3/6 conventions because this geochernical software is the most used one in BRGM. Individual Gibbs free energy and eventually enthalpy and entropy of each species are given depending to the availability of these data. The examination of arsenic contents in various hydrothermal and subsurface wa ...
... the EQ3/6 conventions because this geochernical software is the most used one in BRGM. Individual Gibbs free energy and eventually enthalpy and entropy of each species are given depending to the availability of these data. The examination of arsenic contents in various hydrothermal and subsurface wa ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... face, while others may be from a computer printer. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyrighted material had to be removed, a note will indicate the deletion. Oversize materials (e.g., ...
... face, while others may be from a computer printer. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyrighted material had to be removed, a note will indicate the deletion. Oversize materials (e.g., ...
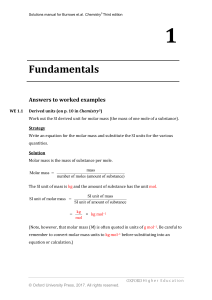
Fundamentals
... WE 1.17 Equilibrium constants in terms of concentrations (on p. 61 in Chemistry3) An equimolar mixture of ethanoic acid and ethanol was heated at 373 K. At equilibrium, the concentration of ethanoic acid was found to be 0.820 mol dm–3. Calculate the concentration of ethyl ethanoate in the equilibriu ...
... WE 1.17 Equilibrium constants in terms of concentrations (on p. 61 in Chemistry3) An equimolar mixture of ethanoic acid and ethanol was heated at 373 K. At equilibrium, the concentration of ethanoic acid was found to be 0.820 mol dm–3. Calculate the concentration of ethyl ethanoate in the equilibriu ...
Influence of Temperature on Electrical
... removes the alkalizing agent and transforms salts into corresponding acids. Consequently, the acid conductivity correlates closely to the contamination. The electrolyte concentrations of the samples are mostly at a lower mg · kg–1 level, equivalent to concentrations of 10–5 to 10–3 mol · L–1. At thi ...
... removes the alkalizing agent and transforms salts into corresponding acids. Consequently, the acid conductivity correlates closely to the contamination. The electrolyte concentrations of the samples are mostly at a lower mg · kg–1 level, equivalent to concentrations of 10–5 to 10–3 mol · L–1. At thi ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.