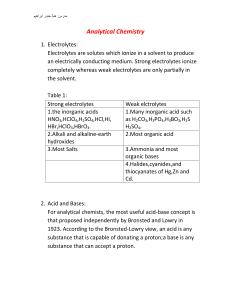
1. (a) Propan-1ol, C2H5CH2OH can be oxidised to propanoic acid
... Many industrial organic reactions produce hydrogen chloride as an additional product. This can be oxidised to chlorine by the Deacon process: 4HCl(g) + O2(g) ...
... Many industrial organic reactions produce hydrogen chloride as an additional product. This can be oxidised to chlorine by the Deacon process: 4HCl(g) + O2(g) ...
Free Energy - Wunder Chem
... LO 5.13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both Ho and So, and calculation or estimation of Go when needed. LO 5.14 The student is able to determine whethe ...
... LO 5.13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both Ho and So, and calculation or estimation of Go when needed. LO 5.14 The student is able to determine whethe ...
1. (a) Write the electronic structure of a manganese atom and a Mn
... The addition of excess aqueous silver nitrate to aqueous solutions of either of these two salts produces a precipitate of silver chloride, AgCl. Ag+(aq) + Cl–(aq) → AgCl(s) Under these conditions all the chloride from the violet salt is precipitated but only two-thirds of the chloride from the green ...
... The addition of excess aqueous silver nitrate to aqueous solutions of either of these two salts produces a precipitate of silver chloride, AgCl. Ag+(aq) + Cl–(aq) → AgCl(s) Under these conditions all the chloride from the violet salt is precipitated but only two-thirds of the chloride from the green ...
Physical chemistry
... 79. Temperature coefficient shows how much ……: A. * The rate of chemical reaction increases for every 10 0C rise in temperature. B. The rate of chemical reaction increases for every 1 0C rise in temperature. C. The rate’s constant increases for every 10 0C rise in temperature. D. The rate’s constant ...
... 79. Temperature coefficient shows how much ……: A. * The rate of chemical reaction increases for every 10 0C rise in temperature. B. The rate of chemical reaction increases for every 1 0C rise in temperature. C. The rate’s constant increases for every 10 0C rise in temperature. D. The rate’s constant ...
The Formation of Solvated Electrons in the Photochemistry of the
... or 1.75 -11H&04 was employed as the solvated electron scavenger, in the presence of 1 M ethanol serving as scavenger for hydrogen atoms formed by the reaction between eaq- and H 3 0 + . ' In both cases the quantum yields for Hzevolution were approximately 0.025, independent of the concentration of H ...
... or 1.75 -11H&04 was employed as the solvated electron scavenger, in the presence of 1 M ethanol serving as scavenger for hydrogen atoms formed by the reaction between eaq- and H 3 0 + . ' In both cases the quantum yields for Hzevolution were approximately 0.025, independent of the concentration of H ...
Briefing Session on 2012 HKDSE Examination (December 2012)
... experiment, calculation of the enthalpy changes of reactions, and calculations on chemical equilibrium. Many candidates were weak in redox chemistry. They were confused about the concepts of oxidation, reduction, oxidizing power, reducing power, position of chemical species in the electrochemic ...
... experiment, calculation of the enthalpy changes of reactions, and calculations on chemical equilibrium. Many candidates were weak in redox chemistry. They were confused about the concepts of oxidation, reduction, oxidizing power, reducing power, position of chemical species in the electrochemic ...
Sample pages 2 PDF
... experimentally determined quantities, (2.8) is called the experimental rate equation. Partial orders x and y usually take the values 1, 2, or 0 (when the rate equation does not depend on the concentration of a particular reactant, its partial order with respect to that reactant is zero). Since the r ...
... experimentally determined quantities, (2.8) is called the experimental rate equation. Partial orders x and y usually take the values 1, 2, or 0 (when the rate equation does not depend on the concentration of a particular reactant, its partial order with respect to that reactant is zero). Since the r ...
Basso08_preprint - University of Strathclyde
... product formation of Z-L-Asp-L-Phe-OMe. This helped to explain both final yields and the enzyme kinetics in this system.57 In such systems salts can be used also when thermodynamics are unfavourable and precipitation is not achieved. The addition of specific counter-ions that form poorly soluble sal ...
... product formation of Z-L-Asp-L-Phe-OMe. This helped to explain both final yields and the enzyme kinetics in this system.57 In such systems salts can be used also when thermodynamics are unfavourable and precipitation is not achieved. The addition of specific counter-ions that form poorly soluble sal ...
Indian National Chemistry Olympiad Theory 2014
... A hydrogen cylinder at 200C has number of moles of hydrogen per unit volume which is (1/100) times the number of moles of hydrogen present in unit volume of Mg2NiH4. Calculate the pressure (in bar) in this cylinder. ...
... A hydrogen cylinder at 200C has number of moles of hydrogen per unit volume which is (1/100) times the number of moles of hydrogen present in unit volume of Mg2NiH4. Calculate the pressure (in bar) in this cylinder. ...
Part II - American Chemical Society
... c. Carbon dioxide (O=C=O) molecules are nonpolar and interact with each other only through weak dispersion forces. These weak forces are easily overcome so CO2 is a gas at room temperature. SiO2 doesn’t have the same molecular formula, because Si does not form double bonds as readily as carbon does. ...
... c. Carbon dioxide (O=C=O) molecules are nonpolar and interact with each other only through weak dispersion forces. These weak forces are easily overcome so CO2 is a gas at room temperature. SiO2 doesn’t have the same molecular formula, because Si does not form double bonds as readily as carbon does. ...
Document
... MiniUnit ~ Solutions (Chapter 15) Introduction and Definitions (Page 451) Solutions are important to chemists because much of the chemistry in the lab, the environment, and within living organisms, occurs in solutions. A brief review of terms: Mixture = ...
... MiniUnit ~ Solutions (Chapter 15) Introduction and Definitions (Page 451) Solutions are important to chemists because much of the chemistry in the lab, the environment, and within living organisms, occurs in solutions. A brief review of terms: Mixture = ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.