Evaluations of pH for Aqueous Solutions
... More accurately, [H+] should be the activity of protons, aH+. To simplify our discussion, we will assume the activity coefficients of all species are one, so activities equal concentrations. Three important equations will be frequently used in the following discussion sections, i.e., ion product of ...
... More accurately, [H+] should be the activity of protons, aH+. To simplify our discussion, we will assume the activity coefficients of all species are one, so activities equal concentrations. Three important equations will be frequently used in the following discussion sections, i.e., ion product of ...
Influence of Ionic Mobile Phase Additives with Low Charge
... selectivity is limited because the hydrophobic moiety of the charged species does not strongly contribute to the analyte retention driving force. ...
... selectivity is limited because the hydrophobic moiety of the charged species does not strongly contribute to the analyte retention driving force. ...
Homogeneous Catalysis
... being measured. To minimize the error this introduces into our measurements, it seems advisable to measure the rate of reaction over periods of time that are short compared with the time it takes for the reaction to occur. We might try, for example, to measure the infinitesimally small change in con ...
... being measured. To minimize the error this introduces into our measurements, it seems advisable to measure the rate of reaction over periods of time that are short compared with the time it takes for the reaction to occur. We might try, for example, to measure the infinitesimally small change in con ...
Nuclear Magnetic Resonance and Potentiometric
... by potentiometry, and the protonation sequence of the various amino and carboxylate groups of NOTA has been studied in DzO as a function of pD from the chemical shifts of the nonlabile protons. Shielding constants for protonation of the amino groups were determined in a NMR study of the triaza macro ...
... by potentiometry, and the protonation sequence of the various amino and carboxylate groups of NOTA has been studied in DzO as a function of pD from the chemical shifts of the nonlabile protons. Shielding constants for protonation of the amino groups were determined in a NMR study of the triaza macro ...
OC 583- ISOTOPE BIGEOCHEMISTRY
... compounds relative to each other. -thus in practice, the absolute ratio of the rare to abundant isotopes is rarely used 3. Important: The value of any standard = 0 ‰. -Thus positive or negative del values imply higher or lower isotope ratios relative to the isotope ratio of the standard 4. The cho ...
... compounds relative to each other. -thus in practice, the absolute ratio of the rare to abundant isotopes is rarely used 3. Important: The value of any standard = 0 ‰. -Thus positive or negative del values imply higher or lower isotope ratios relative to the isotope ratio of the standard 4. The cho ...
Enthalpy change - Don`t Trust Atoms
... Gibbs Free Energy • ΔG represents the Gibbs free energy and combines both enthalpy and entropy. • It is used to determine whether or not a reaction is feasible. ...
... Gibbs Free Energy • ΔG represents the Gibbs free energy and combines both enthalpy and entropy. • It is used to determine whether or not a reaction is feasible. ...
Stable Isotopes in Foraminiferal Carbonate
... (δ45), and the mass 46/44 ratio (δ46), these being the three isotopic masses collected and measured in the analyser. However, the final results required are the δ13C and δ18O ratios relative to the VPDB standard, and a correction for 17O/18O must also be applied. The corrections used in these equati ...
... (δ45), and the mass 46/44 ratio (δ46), these being the three isotopic masses collected and measured in the analyser. However, the final results required are the δ13C and δ18O ratios relative to the VPDB standard, and a correction for 17O/18O must also be applied. The corrections used in these equati ...
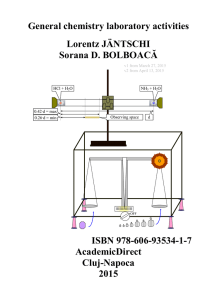
General chemistry laboratory activities, Lorentz
... Figure 2. Reaction flask, distillation arm, reagent flask, and glass tubing Reaction flasks (see Figure 2) are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (suc ...
... Figure 2. Reaction flask, distillation arm, reagent flask, and glass tubing Reaction flasks (see Figure 2) are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (suc ...
Fundamentals of Combustion
... Some general issues . . . . . . . . . . . . . . . . . . . Ideal and non-ideal mixtures . . . . . . . . . . . . . . Ideal mixtures of ideal gases . . . . . . . . . . . . . . 2.3.1 Dalton model . . . . . . . . . . . . . . . . . . 2.3.1.1 Binary mixtures . . . . . . . . . . . 2.3.1.2 Entropy of mixing ...
... Some general issues . . . . . . . . . . . . . . . . . . . Ideal and non-ideal mixtures . . . . . . . . . . . . . . Ideal mixtures of ideal gases . . . . . . . . . . . . . . 2.3.1 Dalton model . . . . . . . . . . . . . . . . . . 2.3.1.1 Binary mixtures . . . . . . . . . . . 2.3.1.2 Entropy of mixing ...
Unit 8: Reactions
... calculating the heat of reaction. 6. Law of Conservation of Mass: Matter may not be created or destroyed by physical or chemical change. This is the basis for balancing chemical reactions. 7. Mole Ratio: The whole-number ratio between components of a balanced chemical reaction. 8. Oxidation: The los ...
... calculating the heat of reaction. 6. Law of Conservation of Mass: Matter may not be created or destroyed by physical or chemical change. This is the basis for balancing chemical reactions. 7. Mole Ratio: The whole-number ratio between components of a balanced chemical reaction. 8. Oxidation: The los ...
Full-text
... The increase in nickel(II) concentration in the feed solution from 5·10-3 to 0.1 M with the cobalt(II) concentration being constant, led to insignificant reduction in the Co(II) flux into the strip solution and cobalt(II) stripping degree, while the nickel(II) stripping degree reduced from 0.6 to 0. ...
... The increase in nickel(II) concentration in the feed solution from 5·10-3 to 0.1 M with the cobalt(II) concentration being constant, led to insignificant reduction in the Co(II) flux into the strip solution and cobalt(II) stripping degree, while the nickel(II) stripping degree reduced from 0.6 to 0. ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.