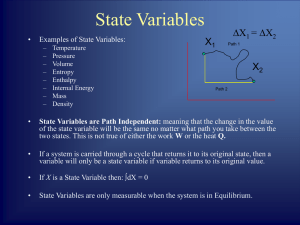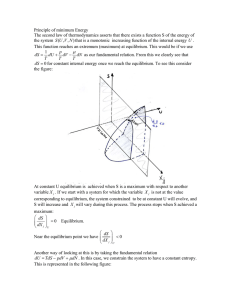
Chemistry: The Molecular Nature of Matter and Change
... 1. Acid-base reactions; concepts of Arrhenius, BrønstedLowry, and Lewis; coordination complexes, amphoterism 2. Precipitation reactions 3. Oxidation-reduction reactions a. Oxidation number b. The role of the electron in oxidation-reduction c. Electrochemistry: electrolytic and galvanic cells; Farada ...
... 1. Acid-base reactions; concepts of Arrhenius, BrønstedLowry, and Lewis; coordination complexes, amphoterism 2. Precipitation reactions 3. Oxidation-reduction reactions a. Oxidation number b. The role of the electron in oxidation-reduction c. Electrochemistry: electrolytic and galvanic cells; Farada ...
Calculating a Ka Value from a Known pH - Chemwiki
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
CE3503 Expectations – Equilibrium Reactions that proceed to
... Reactions that proceed to equilibrium slowly (hours, e.g. BOD exertion, to decades, radioisotope decay) require a kinetic approach as introduced during our discussion of kinetics, reactors and mass balance. Those that proceed more rapidly (milliseconds, e.g. dissociation of strong acid, to minutes, ...
... Reactions that proceed to equilibrium slowly (hours, e.g. BOD exertion, to decades, radioisotope decay) require a kinetic approach as introduced during our discussion of kinetics, reactors and mass balance. Those that proceed more rapidly (milliseconds, e.g. dissociation of strong acid, to minutes, ...
Lecture 10 Activity of chemical components
... The model is valid only in the limit of dilute solution. In general we do not know to calculate the activity coefficients explicitly for other interactions such as hydrogen boding or hydrophobic. Thus a common practice is to set activity coefficients equal to one, implying validity in only dilute so ...
... The model is valid only in the limit of dilute solution. In general we do not know to calculate the activity coefficients explicitly for other interactions such as hydrogen boding or hydrophobic. Thus a common practice is to set activity coefficients equal to one, implying validity in only dilute so ...
ChE 215, Physical Chemistry
... 1) Analysis of Organic Chemical solution using Gas Chromatograph. 2) To study the kinetics of hydrolysis of ethyl acetate solution to ethanol and acetic acid and to determine the reaction rate constant (K). 3) To study the adsorption isotherm using charcoal and acetic acid solution. 4) To determine ...
... 1) Analysis of Organic Chemical solution using Gas Chromatograph. 2) To study the kinetics of hydrolysis of ethyl acetate solution to ethanol and acetic acid and to determine the reaction rate constant (K). 3) To study the adsorption isotherm using charcoal and acetic acid solution. 4) To determine ...
Topics 7 and 17 Outlines
... Obtaining evidence for scientific theories—isotopic labelling and its use in defining equilibrium. (1.8) Common language across different disciplines—the term dynamic equilibrium is used in other contexts, but not necessarily with the chemistry definition in mind. (5.5) Understandings: • A state of ...
... Obtaining evidence for scientific theories—isotopic labelling and its use in defining equilibrium. (1.8) Common language across different disciplines—the term dynamic equilibrium is used in other contexts, but not necessarily with the chemistry definition in mind. (5.5) Understandings: • A state of ...
Chemistry Learning Goals Chap 14 Solutions Minniear
... hydration, diffusion). SWBAT discuss the factors that determine the rate of solution for a solid solute and a liquid solvent (agitation, temperature and surface area). SWBAT explain the effect of temperature on the solubility of a gas. SWBAT explain the processes involved when a solution has reached ...
... hydration, diffusion). SWBAT discuss the factors that determine the rate of solution for a solid solute and a liquid solvent (agitation, temperature and surface area). SWBAT explain the effect of temperature on the solubility of a gas. SWBAT explain the processes involved when a solution has reached ...
Water Chemistry 3
... Thermodynamics tells us where the system should go at equilibrium, and kinetics tells us how fast. • Definition of Equilibrium 1) A system at equilibrium has none of its properties changing with time, no matter how long it is observed 2) A system at equilibrium will return to that state after being ...
... Thermodynamics tells us where the system should go at equilibrium, and kinetics tells us how fast. • Definition of Equilibrium 1) A system at equilibrium has none of its properties changing with time, no matter how long it is observed 2) A system at equilibrium will return to that state after being ...
Equilibrium Constant
... so substitution may be necessary! Must be found experimentally or by means of equilibrium concentrations from thermodynamic data. Also varies with temperature, and constant at a given temperature (just like kinetic rate constants) Independent of initial concentration ...
... so substitution may be necessary! Must be found experimentally or by means of equilibrium concentrations from thermodynamic data. Also varies with temperature, and constant at a given temperature (just like kinetic rate constants) Independent of initial concentration ...
RTF
... Solution: No - this is a very exothermic reaction, releasing a lot of heat (methane, natural gas, is used as a heating fuel). The reverse reaction would be therefore be highly endothermic, requiring a lot of energy to make it go. Thus it is not likely to be reversible. ...
... Solution: No - this is a very exothermic reaction, releasing a lot of heat (methane, natural gas, is used as a heating fuel). The reverse reaction would be therefore be highly endothermic, requiring a lot of energy to make it go. Thus it is not likely to be reversible. ...
Kc and Kp Conversions Hess`s Law in Equilibrium Constants
... to a power that is equal to that number. ...
... to a power that is equal to that number. ...
CHEM121 Exam 4 ObjectivesW16
... -boiling point elevation -freezing/melting point depression -osmotic pressure Titration Lab –find the molarity of an acid Chapter 9: Chemical Reactions (Energy, Rate, Equilibrium) Identify exothermic & endothermic reactions Draw or interpret “energy diagrams” for exothermic & endothermic reactions K ...
... -boiling point elevation -freezing/melting point depression -osmotic pressure Titration Lab –find the molarity of an acid Chapter 9: Chemical Reactions (Energy, Rate, Equilibrium) Identify exothermic & endothermic reactions Draw or interpret “energy diagrams” for exothermic & endothermic reactions K ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.