Le Chatelier`s Principle Quiz Answer Key
... If the statement is true, write "true"on your answer sheet. If it is false, change the underlined word or words to make the statement true and write the corrected answer on your answer sheet. NH4Cl(s) + heat NH3(g) + HCl(g) 5. The above reaction is exothermic. 6. The production of ammonia from amm ...
... If the statement is true, write "true"on your answer sheet. If it is false, change the underlined word or words to make the statement true and write the corrected answer on your answer sheet. NH4Cl(s) + heat NH3(g) + HCl(g) 5. The above reaction is exothermic. 6. The production of ammonia from amm ...
Lecture 21 revised (Slides) October 12
... Transition Metals – Electron Configurations • Transition metals have surprisingly rich chemistry. The electronic configurations of the neutral atoms are relatively complex since d subshells (with 5 orbitals) are being filled. For the first series of transition metals the 4s and 3d subshells have si ...
... Transition Metals – Electron Configurations • Transition metals have surprisingly rich chemistry. The electronic configurations of the neutral atoms are relatively complex since d subshells (with 5 orbitals) are being filled. For the first series of transition metals the 4s and 3d subshells have si ...
Introductory Chemistry Test Review
... 9. For the following chemical compounds, predict whether each will be soluble or insoluble in aqueous solution. a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 ...
... 9. For the following chemical compounds, predict whether each will be soluble or insoluble in aqueous solution. a. Al(OH)3 b. Hg2Cl2 c. (NH4)2CO3 10. For the following aqueous chemical reactions, predict the possible products and identify any products that will be insoluble. a. CaCl2 + K2S b. MgCl2 ...
Types of Reactions
... Take out a sheet of notebook paper and your drills. For each date listed, write the question AND the answer. You have 10 minutes. ...
... Take out a sheet of notebook paper and your drills. For each date listed, write the question AND the answer. You have 10 minutes. ...
Ch 19 test_take-home
... 19) Which one of the following processes produces a decrease in the entropy of the system? A) boiling water to form steam B) dissolution of solid KCl in water C) mixing of two gases into one container D) freezing water to form ice E) melting ice to form water 20) For a reaction to be spontaneous und ...
... 19) Which one of the following processes produces a decrease in the entropy of the system? A) boiling water to form steam B) dissolution of solid KCl in water C) mixing of two gases into one container D) freezing water to form ice E) melting ice to form water 20) For a reaction to be spontaneous und ...
Synthesis of Alum Lab
... NH3 is added to Cu2+: Cu(NH3)42+ cupric tetraamine NH3 is added to Ag+: Ag(NH3)2+ silver diamine Conc. OH- is added to Zn(OH)2: Zn(OH)42+ Fe3+ in thiocyanate (SCN-): Fe(SCN)63- complex Co2+ with chlorine: CoCl42Al oxidized in base: Al(OH)4- ...
... NH3 is added to Cu2+: Cu(NH3)42+ cupric tetraamine NH3 is added to Ag+: Ag(NH3)2+ silver diamine Conc. OH- is added to Zn(OH)2: Zn(OH)42+ Fe3+ in thiocyanate (SCN-): Fe(SCN)63- complex Co2+ with chlorine: CoCl42Al oxidized in base: Al(OH)4- ...
CHEMISTRY 102B Name Hour Exam II March 19, 2015 Signature
... b) The ground state electron configuration for the most stable ion of sodium in a compound is 1s22s22p 6. c) The ground state electron configuration for the valence electrons of the halogens (Group 7A) is ns2np5. d) At least two of the above statements (a-c) are false. e) All of the above statements ...
... b) The ground state electron configuration for the most stable ion of sodium in a compound is 1s22s22p 6. c) The ground state electron configuration for the valence electrons of the halogens (Group 7A) is ns2np5. d) At least two of the above statements (a-c) are false. e) All of the above statements ...
Lecture 7. Fundamentals of atmospheric chemistry: Part 2 1
... These terms are sometimes confusing since the reduction process involves adding an electron. Keep in mind it's the charge that's being reduced in this case. Oxidation receives its name because almost all reactions with oxygen involve some other element losing electrons to the oxygen. Only fluorine w ...
... These terms are sometimes confusing since the reduction process involves adding an electron. Keep in mind it's the charge that's being reduced in this case. Oxidation receives its name because almost all reactions with oxygen involve some other element losing electrons to the oxygen. Only fluorine w ...
Chapter 3. Analysis of Environmental System 3.1 Analysis of a
... Reaction equation (3.2.1) means that a moles of material A and b moles of material B react chemically to produce c moles of material C, and d moles of material D. At the same time, c moles of material C and d moles of material D react to produce a mole of material A, and b moles of material B. To un ...
... Reaction equation (3.2.1) means that a moles of material A and b moles of material B react chemically to produce c moles of material C, and d moles of material D. At the same time, c moles of material C and d moles of material D react to produce a mole of material A, and b moles of material B. To un ...
Ab initio studies on optimized geometries for the thiazole
... that charge transfer occurs within the molecule. The geometries and energies obtained from HF/6-31G* calculations are in good agreement with the experimentally observed data. Keywords: N,N’-bis(2-Thiazol-yl)methylenediamin, HF, HOMO, LOMO. ____________________________________________________________ ...
... that charge transfer occurs within the molecule. The geometries and energies obtained from HF/6-31G* calculations are in good agreement with the experimentally observed data. Keywords: N,N’-bis(2-Thiazol-yl)methylenediamin, HF, HOMO, LOMO. ____________________________________________________________ ...
RXN-4-STUDENTS - Rothschild Science
... element you have NH3 (one nitrogen, three hydrogen)- DON’T mess with these!! Coefficients – small whole number that appears ...
... element you have NH3 (one nitrogen, three hydrogen)- DON’T mess with these!! Coefficients – small whole number that appears ...
Catalytic Synthesis of Organophosphorus Compounds from
... POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, the elaboration of new environmentally acceptable processes for the syntheses of organophosphorus compo ...
... POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, the elaboration of new environmentally acceptable processes for the syntheses of organophosphorus compo ...
AP Reactions - Georgetown ISD
... When the oxides of an alkali metal (Family 1), Ca, Ba, or Sr dissolve in water, hydroxides will form, but no gases will be released. Example: K2O(s) + H2O ...
... When the oxides of an alkali metal (Family 1), Ca, Ba, or Sr dissolve in water, hydroxides will form, but no gases will be released. Example: K2O(s) + H2O ...
Gas and Vapor Phase Explosions
... 3. Find the spark coil and turn it on by rotating the knob on its bottom clockwise. You will hear it begin to make a sparking sound. CAUTION: Do not touch the tip as you will get a shock from it! 4. Touch one of the screws in the polyethylene bottle with the tip of the spark coil and stand back! 5. ...
... 3. Find the spark coil and turn it on by rotating the knob on its bottom clockwise. You will hear it begin to make a sparking sound. CAUTION: Do not touch the tip as you will get a shock from it! 4. Touch one of the screws in the polyethylene bottle with the tip of the spark coil and stand back! 5. ...
Equilibrium
... X=Bonding Atom E=Electron Pair ● When molecules exhibit resonance, any structures can be used to predict molecular structure using VSEPR model ● VSEPR works in most cases for non-ionic compounds Sigma and pi bonds ● Sigma Bonds: Bond in which the electron pair is shared in an area centered on a line ...
... X=Bonding Atom E=Electron Pair ● When molecules exhibit resonance, any structures can be used to predict molecular structure using VSEPR model ● VSEPR works in most cases for non-ionic compounds Sigma and pi bonds ● Sigma Bonds: Bond in which the electron pair is shared in an area centered on a line ...
Week 7 - Acid-base, redox
... Answers: For PH3, assign H=+1. The three bound H atoms give a total of +3. The algebraic sum of P + 3 = 0, giving P= −3. For BrO3−, assign O=-2. The three bound O atoms give a total of -6. The algebraic sum of Br + -6 = -1, giving Br=+5. For H2SO4, assign H=+1 and O=-2. The two bound hydrogen atoms ...
... Answers: For PH3, assign H=+1. The three bound H atoms give a total of +3. The algebraic sum of P + 3 = 0, giving P= −3. For BrO3−, assign O=-2. The three bound O atoms give a total of -6. The algebraic sum of Br + -6 = -1, giving Br=+5. For H2SO4, assign H=+1 and O=-2. The two bound hydrogen atoms ...
Chemical Reactions - Johnston County Schools
... Substances other than hydrocarbons can also combust. However, you may not be able to tell whether it’s combustion from the chemical equation alone. Remember that combustion must have O2 as a reactant and must release (exothermic) heat and light energy. Reactions with O2.mov ...
... Substances other than hydrocarbons can also combust. However, you may not be able to tell whether it’s combustion from the chemical equation alone. Remember that combustion must have O2 as a reactant and must release (exothermic) heat and light energy. Reactions with O2.mov ...
Dr. Baxley`s Thermodynamics Worksheet
... b) ClO2−(aq) < ClO3−(aq) < ClO4−(aq) c) C6H6 < C7H14 < C7H16 d) PF3(g) < PF5(g) < PF2Cl3(g) 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous ...
... b) ClO2−(aq) < ClO3−(aq) < ClO4−(aq) c) C6H6 < C7H14 < C7H16 d) PF3(g) < PF5(g) < PF2Cl3(g) 4. This is when a spontaneous reaction has a + ∆H and a + ∆S. The reaction is spontaneous because of its ∆S, so ∆S drives the reaction. 5. a. balance it first! ∆S is probably − because there are more gaseous ...
Solutions of Chapter 4 4.1. Compare light microscopy, transmission
... Unfortunately, reduction of probe size is accompanied by probe current reduction. To obtain the best resolution, we should select a short work distance, a medium or small size of final aperture, a high acceleration voltage, and correct astigmatism. Also, we should adjust the probe current, which is ...
... Unfortunately, reduction of probe size is accompanied by probe current reduction. To obtain the best resolution, we should select a short work distance, a medium or small size of final aperture, a high acceleration voltage, and correct astigmatism. Also, we should adjust the probe current, which is ...
Section 3.6
... (c) A magnet should separate these coins easily, because nickel is ferromagnetic (strongly magnetic) and silver is not. 17. ESR spectroscopy places samples of paramagnetic material in a high uniform magnetic field to split the energy levels of the ground state. The results can be used to help determ ...
... (c) A magnet should separate these coins easily, because nickel is ferromagnetic (strongly magnetic) and silver is not. 17. ESR spectroscopy places samples of paramagnetic material in a high uniform magnetic field to split the energy levels of the ground state. The results can be used to help determ ...
in a Chemical Reactor - Max-Planck
... and clean it. This is necessary because a chemical reaction rarely delivers the desired compound exclusively. Quite apart from this, reaction mixtures often contain catalysts or other additives, and the solvents in which most reactions occur also have to be removed. Thus, as a possible method of pro ...
... and clean it. This is necessary because a chemical reaction rarely delivers the desired compound exclusively. Quite apart from this, reaction mixtures often contain catalysts or other additives, and the solvents in which most reactions occur also have to be removed. Thus, as a possible method of pro ...
FE Review Chemistry - UTSA College of Engineering
... 2. Which statement is incorrect? a) An element may be separated into atoms b) An element may be a gas, a liquid, or a solid c) A compound can be separated into its elements by chemical means d) An element is always heterogeneous e) A compound may be a gas, a liquid, or a solid 3. In relation to the ...
... 2. Which statement is incorrect? a) An element may be separated into atoms b) An element may be a gas, a liquid, or a solid c) A compound can be separated into its elements by chemical means d) An element is always heterogeneous e) A compound may be a gas, a liquid, or a solid 3. In relation to the ...
oxidation and reduction
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
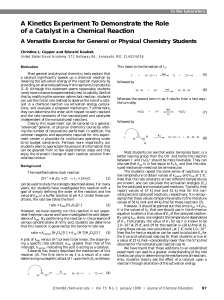
JCE0198 p0087 A Kinetics Experiment To Demonstrate the Role of
... better leaving group than the OH{ and hence the reaction between I{ and H3O 2+ should be more favorable. They can also see that kcat is in fact equal to K1 kH and that the catalyzed mechanism need not be termolecular. The students repeat the same series of reactions at a low temperature to obtain va ...
... better leaving group than the OH{ and hence the reaction between I{ and H3O 2+ should be more favorable. They can also see that kcat is in fact equal to K1 kH and that the catalyzed mechanism need not be termolecular. The students repeat the same series of reactions at a low temperature to obtain va ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.