When Gold Is Not Noble: Nanoscale Gold
... vibrational frequency and linewidth of adsorbed CO are rather sensitive to the size and structure of the cluster). In the absence of oxygen, CO desorbs from the supported Au8 cluster between 150 and 180 K. The catalytically formed 13C16O18O desorbs already at 140 K with the main desorption peak at 2 ...
... vibrational frequency and linewidth of adsorbed CO are rather sensitive to the size and structure of the cluster). In the absence of oxygen, CO desorbs from the supported Au8 cluster between 150 and 180 K. The catalytically formed 13C16O18O desorbs already at 140 K with the main desorption peak at 2 ...
Spring 2014
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
School of Chemistry and Physics Westville Campus, Durban
... Electronegativity increases from left to right along a row of the periodic table, and stays the same from top to bottom within a group ...
... Electronegativity increases from left to right along a row of the periodic table, and stays the same from top to bottom within a group ...
A) Sn4+ → Sn2+ + 2e
... 76. In the electrolysis of aqueous copper (II) bromide solution, CuBr 2(aq), 1.00 gram of Cu is deposited at the cathode. How many grams of bromine are formed at the ...
... 76. In the electrolysis of aqueous copper (II) bromide solution, CuBr 2(aq), 1.00 gram of Cu is deposited at the cathode. How many grams of bromine are formed at the ...
Chemistry 400
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
... 8) Choose the transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength photon. A) n = 1 to n = 2 B) n = 2 to n = 3 C) n = 4 to n = 5 D) n = 6 to n = 3 E) n = 3 to n = 1 9) Which of the following statements is TRUE? A) We can sometimes know the exact location an ...
UNSYMMETRICAL DINUCLEAR RHODIUM COMPLEXES WITH
... arsanylarylthiolates AsS–, AsS22– and AsS33– is less well developed. Although a number of examples of transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligan ...
... arsanylarylthiolates AsS–, AsS22– and AsS33– is less well developed. Although a number of examples of transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligan ...
Unit 9 - Kinetics and Equilibrium
... It is a fraction with the concentrations of reactants and products expressed in moles per liter Each concentration is then raised to the power of its coefficient in a balanced equation. The expression equals a value called the equilibrium constant Keq, which remains the same for a particular r ...
... It is a fraction with the concentrations of reactants and products expressed in moles per liter Each concentration is then raised to the power of its coefficient in a balanced equation. The expression equals a value called the equilibrium constant Keq, which remains the same for a particular r ...
AP Chemistry: Total Notes Review
... 3: Fill the octets 4: use double and triple bonds as necessary o Formal charges: subtract the amount of electrons on the periodic table (for that element) from the electrons you drew in ~ 0 means right on ~ the negative charge should be on the most electronegative atom o Resonance: when one Lewis st ...
... 3: Fill the octets 4: use double and triple bonds as necessary o Formal charges: subtract the amount of electrons on the periodic table (for that element) from the electrons you drew in ~ 0 means right on ~ the negative charge should be on the most electronegative atom o Resonance: when one Lewis st ...
Chapter 4: Aqueous Reactions and Solution Stoichiometry
... oxidation number of −1 when they are negative; they can have positive oxidation numbers, however, most notably in oxyanions. ...
... oxidation number of −1 when they are negative; they can have positive oxidation numbers, however, most notably in oxyanions. ...
Section 2 Oxidation Numbers
... • In general when assigning oxidation numbers, shared electrons are assumed to “belong” to the more electronegative atom in each bond. • More-specific rules are provided by the following guidelines. ...
... • In general when assigning oxidation numbers, shared electrons are assumed to “belong” to the more electronegative atom in each bond. • More-specific rules are provided by the following guidelines. ...
Equilibrium Electrochemistry
... reaction drives electrons through an external circuit. the work that a given transfer of electrons can accomplish depends on the potential difference between the two electrodes. This potential differences is called the cell potential and is measured in volts, V (1 V = 1 JC-1 s). A cell in whic ...
... reaction drives electrons through an external circuit. the work that a given transfer of electrons can accomplish depends on the potential difference between the two electrodes. This potential differences is called the cell potential and is measured in volts, V (1 V = 1 JC-1 s). A cell in whic ...
Gas phase chemistry of neutral metal clusters
... or troughs were likely to be the locus of enhanced catalytic activity. Catalytic properties of a material (activity, selectivity, and stability) are, in general, determined by chemical (electronic) properties of surface atoms/molecules [11,12]. On the basis of the concept that a catalytic reaction o ...
... or troughs were likely to be the locus of enhanced catalytic activity. Catalytic properties of a material (activity, selectivity, and stability) are, in general, determined by chemical (electronic) properties of surface atoms/molecules [11,12]. On the basis of the concept that a catalytic reaction o ...
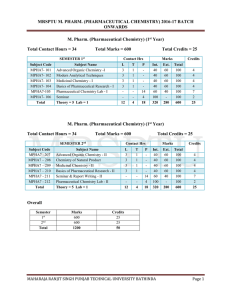
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
Chemistry
... tetrahedral coordination entities using energy level diagram and their comparison. Spectrochemical series, compare weak field ligand – strong field ligand with respect to d4 ions in octahedral field ( 0 < P, 0 > P). Explanation for colour of complexes using CFT, examples. Isomerism: Structural – lin ...
... tetrahedral coordination entities using energy level diagram and their comparison. Spectrochemical series, compare weak field ligand – strong field ligand with respect to d4 ions in octahedral field ( 0 < P, 0 > P). Explanation for colour of complexes using CFT, examples. Isomerism: Structural – lin ...
Problem 14. MAGNESIUM DETERMINATION
... The first Periodic system listed 66 elements only, of which three were unknown. In the present-day system there are 118 elements. The last, 118 th element was discovered in 2005 during the collaborative studies by the Joint Institute for Nuclear Research (Russia) and the Livermore National Laborator ...
... The first Periodic system listed 66 elements only, of which three were unknown. In the present-day system there are 118 elements. The last, 118 th element was discovered in 2005 during the collaborative studies by the Joint Institute for Nuclear Research (Russia) and the Livermore National Laborator ...
Size-Selective Hydrogenation of Olefins by Dendrimer
... particularly attractive for catalysis. First, solubility is controlled principally by the chemical composition of the dendrimer periphery.1 Second, because the encapsulated particles are very small (typically 1-3 nm),1 they have a high surface-area-tovolume ratio, which is important for high efficie ...
... particularly attractive for catalysis. First, solubility is controlled principally by the chemical composition of the dendrimer periphery.1 Second, because the encapsulated particles are very small (typically 1-3 nm),1 they have a high surface-area-tovolume ratio, which is important for high efficie ...
Chemistry - Textbooks Online
... From the study of quantum numbers, various rules are put forward for filling of electrons in various orbitals by following ...
... From the study of quantum numbers, various rules are put forward for filling of electrons in various orbitals by following ...
November 2016 (v3) QP - Paper 4 CIE Chemistry A-level
... (f) Iron(III) ions can oxidise vanadium metal. Construct an equation for the reaction of an excess of iron(III) ions with vanadium metal. Use of the Data Booklet will be helpful. ...
... (f) Iron(III) ions can oxidise vanadium metal. Construct an equation for the reaction of an excess of iron(III) ions with vanadium metal. Use of the Data Booklet will be helpful. ...
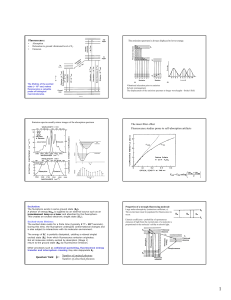
Fluorescence: Fluorescence studies prone to self
... The flurophore exists in some ground state (S0). A photon of energy hvEX is supplied by an external source such as an incandescent lamp or a laser and absorbed by the fluorophore. This creates an excited electronic singlet state (S1'). Excited-state lifetime: The excited state exists for a finite ti ...
... The flurophore exists in some ground state (S0). A photon of energy hvEX is supplied by an external source such as an incandescent lamp or a laser and absorbed by the fluorophore. This creates an excited electronic singlet state (S1'). Excited-state lifetime: The excited state exists for a finite ti ...
November 2016 (v1) QP - Paper 4 CIE Chemistry A-level
... (f) Iron(III) ions can oxidise vanadium metal. Construct an equation for the reaction of an excess of iron(III) ions with vanadium metal. Use of the Data Booklet will be helpful. ...
... (f) Iron(III) ions can oxidise vanadium metal. Construct an equation for the reaction of an excess of iron(III) ions with vanadium metal. Use of the Data Booklet will be helpful. ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... reacting element but less reactive. The similarity can be as fundamental as belonging to the same chemical family, or as loose as both having the same charge polarity in compounds. In any case, the more reactive element takes the place of the less active element in the compound and the less active e ...
... reacting element but less reactive. The similarity can be as fundamental as belonging to the same chemical family, or as loose as both having the same charge polarity in compounds. In any case, the more reactive element takes the place of the less active element in the compound and the less active e ...
Writing Chemical Reactions
... reacting element but less reactive. The similarity can be as fundamental as belonging to the same chemical family, or as loose as both having the same charge polarity in compounds. In any case, the more reactive element takes the place of the less active element in the compound and the less active e ...
... reacting element but less reactive. The similarity can be as fundamental as belonging to the same chemical family, or as loose as both having the same charge polarity in compounds. In any case, the more reactive element takes the place of the less active element in the compound and the less active e ...
Fall 2013 Final practice questions w/o solution
... E) Once an electron is promoted from one energy level to another, it is "stuck" there and cannot return to the ground state. ...
... E) Once an electron is promoted from one energy level to another, it is "stuck" there and cannot return to the ground state. ...
No Slide Title - McMaster Chemistry
... conjugate BASE are both present at the same time WEAK ACID: (acetic acid a.k.a. vinegar) CH3CO2H + H2O CH3CO2- (aq) + H3O+ (aq) WEAK BASE: NH3 (g) + H2O NH4+ (aq) + OH- (aq) 1A03/1E03 Types of Reactions (2) ...
... conjugate BASE are both present at the same time WEAK ACID: (acetic acid a.k.a. vinegar) CH3CO2H + H2O CH3CO2- (aq) + H3O+ (aq) WEAK BASE: NH3 (g) + H2O NH4+ (aq) + OH- (aq) 1A03/1E03 Types of Reactions (2) ...
Photoredox catalysis
_Schematic.png?width=300)
Photoredox catalysis is a branch of catalysis that harnesses the energy of visible light to accelerate a chemical reaction via a single-electron transfer. This area is named as a combination of ""photo-"" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would not react. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While each class of materials has advantages, soluble transition-metal complexes are used most often.Study of this branch of catalysis led to the development of new methods to accomplish known and new chemical transformations. One attraction to the area is that photoredox catalysts are often less toxic than other reagents often used to generate free radicals, such as organotin reagents. Furthermore, while photoredox catalysts generate potent redox agents while exposed to light, they are innocuous under ordinary conditions Thus transition-metal complex photoredox catalysts are in some ways more attractive than stoichiometric redox agents such as quinones. The properties of photoredox catalysts can be modified by changing ligands and the metal, reflecting the somewhat modular nature of the catalyst.While photoredox catalysis has most often been applied to generate known reactive intermediates in a novel way, the study of this mode of catalysis led to the discovery of new organic reactions, such as the first direct functionalization of the β-arylation of saturated aldehydes. Although the D3-symmetric transition-metal complexes used in many photoredox-catalyzed reactions are chiral, the use of enantioenriched photoredox catalysts led to low levels of enantioselectivity in a photoredox-catalyzed aryl-aryl coupling reaction, suggesting that the chiral nature of these catalysts is not yet a highly effective means of transmitting stereochemical information in photoredox reactions. However, while synthetically useful levels of enantioselectivity have not been achieved using chiral photoredox catalysts alone, optically-active products have been obtained through the synergistic combination of photoredox catalysis with chiral organocatalysts such as secondary amines and Brønsted acids.